|
Evidence for Evolution and an Old Earth, a Catholic
Perspective (updated December 2008)
"According to the widely accepted scientific
account, the universe erupted 15 billion years ago in an explosion
called the 'Big Bang' and has been expanding and cooling ever since.
Later there gradually emerged the conditions necessary for the formation
of atoms, still later the condensation of galaxies and stars, and about
10 billion years later the formation of planets. In our own solar system
and on earth (formed about 4.5 billion years ago), the conditions have
been favorable to the emergence of life. While there is little consensus
among scientists about how the origin of this first microscopic life is
to be explained, there is general agreement among them that the first
organism dwelt on this planet about 3.5 - 4 billion years ago. Since it
has been demonstrated that all living organisms on earth are genetically
related, it is virtually certain that all living organisms have
descended from this first organism. Converging evidence from many
studies in the physical and biological sciences furnishes mounting
support for some theory of evolution to account for the development and
diversification of life on earth, while controversy continues over the
pace and mechanisms of evolution. While the story of human origins is
complex and subject to revision, physical anthropology and molecular
biology combine to make a convincing case for the origin of the human
species in Africa about 150,000 years ago in a humanoid population of
common genetic lineage. However it is to be explained, the decisive
factor in human origins was a continually increasing brain size,
culminating in that of homo sapiens. With the development of the human
brain, the nature and rate of evolution were permanently altered: with
the introduction of the uniquely human factors of consciousness,
intentionality, freedom and creativity, biological evolution was recast
as social and cultural evolution." (From the International
Theological Commission, headed by then Joseph Cardinal Ratzinger now
Pope Benedict XVI, statement "Communion
and Stewardship: Human Persons Created in the Image of God,"
plenary sessions held in Rome 2000-2002, published July 2004)
The purpose of this article is to reveal
the scientific evidence in favor of an old earth and (more
controversial) macroevolution (defined as "the theory of universal
common descent with gradual modification"). Much of my material
I have borrowed from the comprehensive TalkOrigins.org
site, as well as the books I have listed below. I will be quoting a
young-earth Catholic creationist (who takes the first chapters of the book of Genesis quite
literally) and respond to some of his criticisms
and confusion over science, Catholic theology, and the Bible.
see also Part 1: The Scientific Evidence for an Old Earth
 The Evidence for Evolution The Evidence for Evolution
Transitional Fossils
(below, being updated)
Reply to a Catholic
Creationist (below, updated Dec 2008)
Bibliography (updated July
2010)
Definition of Evolution
Let's remember what Pope John Paul II stated to the Pontifical
Academy of Sciences in 1996.
"Today, almost half a century after the
publication of the [Pope Pius XII Humani Generis] Encyclical, new
knowledge has led to the recognition of more than a hypothesis in the
theory of evolution. It is indeed remarkable that this theory has been
progressively accepted by researchers, following a series of discoveries
in various fields of knowledge. The convergence, neither sought
nor fabricated, of the results of work that was conducted independently
is in itself a significant argument in favor of this theory."
Now let's find out why he might say this. What is the scientific
evidence for macroevolution? First, a simple definition:
"Macroevolution is the theory of universal common descent with
gradual modification." Or as Douglas Theobald in his "29+
Evidences for Macroevolution" further defines:
"Common descent is a general descriptive theory
that proposes to explain the origins of living organisms....Because it
is so well supported scientifically, macroevolution is often called the
'fact of evolution' by biologists. The theory specifically postulates
that all of the earth's known biota are genealogically related, much in
the same way that siblings or cousins are related to one another. Thus,
macroevolutionary processes necessarily entail the transformation of one
species into another and, consequently, the origin of higher taxa."
Stephen Jay Gould of Harvard explains why evolution is considered both
a fact and a theory.
"Well evolution is a theory. It is also a fact.
And facts and theories are different things, not rungs in a hierarchy of
increasing certainty. Facts are the world's data. Theories are
structures of ideas that explain and interpret facts. Facts don't go
away when scientists debate rival theories to explain them. Einstein's
theory of gravitation replaced Newton's in this century, but apples
didn't suspend themselves in midair, pending the outcome....In science
"fact" can only mean 'confirmed to such a degree that it would
be perverse to withhold provisional consent.' I suppose that apples
might start to rise tomorrow, but the possibility does not merit equal
time in physics classrooms. Evolutionists have been very clear about
this distinction of fact and theory from the very beginning, if only
because we have always acknowledged how far we are from completely
understanding the mechanisms (theory) by which evolution (fact)
occurred." (Stephen Jay Gould "Evolution as Fact and
Theory" in Discover magazine, May 1981)
This article will lay out some of the best evidence for macroevolution while
not delving into natural selection, punctuated equilibrium, or the various proposed
"mechanisms" of
evolution. In Finding Darwin's God (1999) Kenneth Miller has written a fabulous book that
presents the
evidence for evolution and responds to prominent critics from various
perspectives (young-earth creationists represented by Duane Gish/Henry
Morris/ICR in the chapter "God the Charlatan"; progressive
creationists like Phillip E. Johnson in "God the Magician"; and
intelligent design advocates like Michael Behe in
"God the Mechanic"). Dr. Miller says to deny the evidence for
evolution that we see from natural history and propose
"intelligent design" in its place is to posit a Creator who
mimics evolution.
 "Is it any wonder that biologists are unable to
take intelligent design seriously? Over and over again, the imposition
of intelligent design on the facts of natural history requires us to
imagine a designer who creates successive forms that mimic evolution.
Magicians are master illusionists, and if this magical designer had
anything in mind, it must have been to cast the illusion of evolution
and nothing else....Like it or not, intelligent design requires us to
believe that the past was a time of magic in which species appeared
out of nothing. That magic began with the dawn of life on this planet,
and continued unabated for more than a billion years, bringing a grand
parade of living things into existence. Throughout this time, novel
organisms sprang into existence one after another, transforming the
earth and producing eras in which organisms now long extinct dominated
the planet." (Kenneth Miller, from chapter 4 "God the
Magician" in Finding Darwin's God, page 99, 100) "Is it any wonder that biologists are unable to
take intelligent design seriously? Over and over again, the imposition
of intelligent design on the facts of natural history requires us to
imagine a designer who creates successive forms that mimic evolution.
Magicians are master illusionists, and if this magical designer had
anything in mind, it must have been to cast the illusion of evolution
and nothing else....Like it or not, intelligent design requires us to
believe that the past was a time of magic in which species appeared
out of nothing. That magic began with the dawn of life on this planet,
and continued unabated for more than a billion years, bringing a grand
parade of living things into existence. Throughout this time, novel
organisms sprang into existence one after another, transforming the
earth and producing eras in which organisms now long extinct dominated
the planet." (Kenneth Miller, from chapter 4 "God the
Magician" in Finding Darwin's God, page 99, 100)
It should be noted that the biologist Miller considers himself an
"orthodox Catholic" as well as an "orthodox
Darwinist" (from his appearance on the 2001
PBS special on evolution). He sees no problem reconciling his
Catholic faith with the Darwinian theory of evolution.
Another Catholic writing on evolutionary theory, but from the
"intelligent design" standpoint, is Michael Behe, the
biochemist from Lehigh University.  Young-earth
creationists (whether Catholic or evangelical), thinking they had an ally in their fight
against science, were no doubt very disappointed when they
read this from Behe at the beginning of his book (supposedly)
challenging evolution: Young-earth
creationists (whether Catholic or evangelical), thinking they had an ally in their fight
against science, were no doubt very disappointed when they
read this from Behe at the beginning of his book (supposedly)
challenging evolution:
"Evolution is a controversial topic, so it is
necessary to address a few basic questions at the beginning of the
book. Many people think that questioning Darwinian evolution must be
equivalent to espousing creationism. As commonly understood,
creationism involves belief in an earth formed only about ten thousand
years ago, an interpretation of the Bible that is still very popular. For
the record, I have no reason to doubt that the universe is the
billions of years old that physicists say it is. Further, I find the
idea of common descent (that all organisms share a common ancestor)
fairly convincing, and have no particular reason to doubt it.
I greatly respect the work of my colleagues who study the development
and behavior of organisms within an evolutionary framework, and I
think that evolutionary biologists have contributed enormously to our
understanding of the world. Although Darwin's mechanism -- natural
selection working on variation -- might explain many things, however,
I do not believe it explains molecular life." (Michael Behe, Darwin's
Black Box, page 5)
So Behe himself does not doubt evolution (descent with modification
from a common ancestor), he challenges the idea that the current mechanisms of
evolution can explain the "irreducible complexity" of life at the molecular
level. I don't intend to critique Behe's theory of "intelligent design."
The purpose of this article is to lay out the basic scientific evidence for an
old earth and macroevolution. Kenneth Miller has critiqued Behe's book (see
chapter 5 of Finding Darwin's God, "God the
Mechanic") and Behe has responded (see Behe's appendix in Science
and Evidence for Design in the Universe titled "Answering Scientific Criticisms of Intelligent Design" and
the
Access Research Network Behe page online). See also Kenneth
Miller's Evolution
Page at Brown University. One can read the
replies back and forth from both sides and decide if "intelligent
design" is a valid argument against evolution.
Evidence for Macroevolution
Now I will outline
some of the evidence for macroevolution.
Adapted from the detailed TalkOrigins "29+ Evidences for
Macroevolution" (from November 2002 version) by Douglas Theobald, Ph.D.
Universal common descent is the hypothesis that all living organisms
are the lineal descendants of one original living species. All the
diversity of life, both past and present, was originated by normal
reproductive processes observable today. Thus, all extant species are
related in a strict genealogical sense. More specifically,
macroevolution is proposed to occur on a geological timescale and in a
gradual manner. "Gradualness" has little to do with the rate
or tempo of evolution; it is a mode of change that is dependent on
population phenomena. The truth of macroevolution is not assumed a
priori in this discussion. Simply put, the hypothesis of common descent,
combined with modern biological knowledge, is used to deduce
predictions; these predictions are then compared to the real world in
order see how the hypothesis fairs in light of the observable evidence.
Without assuming the truth of universal common descent, it is highly
probable that the hypothesis will indeed fail for most of these
predictions -- and this is exactly why many of these predictions are
such strong evidence for common descent.
The Unique Universal Phylogenetic Tree
Descent from a common ancestor entails a process of branching and
divergence of species, in common with any genealogical process. The
macroevolutionary prediction of a unique, historical universal
phylogenetic tree (the "Tree of Life") is the most important,
powerful, and basic conclusion from the hypothesis of universal common
descent. If modern species have descended from ancestral ones in this
tree-like, branching manner, a rigorous classification of species should
reflect their divergence and it should be possible to infer the true
historical tree that traces their paths of descent. Cladistics is a
method used to determine the standard phylogenetic tree based on
morphology by classifying organisms according to their shared derived
characters (proposed by taxonomist Willi Hennig in 1950).
According to the theory of common descent, modern living organisms,
with all their incredible differences, are the progeny of one single
species in the distant past. In spite of the extensive variation of form
and function among organisms, several fundamental criteria characterize
all life: (1) replication, (2) information flow in continuity of kind,
(3) catalysis, and (4) energy utilization (metabolism). These four
functions are required to generate a physical historical process that
can be described by a phylogenetic tree.
A basic prediction of the genealogical relatedness of all life,
combined with the constraint of gradualism, is that organisms should be
very similar in the particular mechanisms and structures that execute
these four basic life processes. All known living things use polymers to
perform these four basic functions: polynucleotides, polypeptides, and
polysaccharides. All known life uses the same polymer, polynucleotide
(DNA or RNA), for storing species specific information. All known
organisms base replication on the duplication of this molecule. In all
known organisms, enzymatic catalysis is based on the abilities provided
by protein molecules which are constructed with the same subset of 22
amino acids (even though there are 293 naturally occurring amino
acids). All known organisms, with extremely rare exceptions, use
the same genetic code for transmitting information from the genetic
material to the catalytic material. All known organisms use extremely
similar, if not the same, metabolic pathways and metabolic enzymes in
processing energy-containing molecules.

If there is one historical phylogenetic tree which unites
all species in an objective genealogy, all separate lines of evidence
should converge on the same tree. And indeed, independently derived
phylogenetic trees of all organisms match each other with an extremely
high degree of statistical significance. There are over 1041 different
possible ways to arrange the 30 major taxa represented into a phylogenetic
tree (picture above). Speaking quantitatively, independent
morphological and molecular measurements have determined
the standard phylogenetic tree to better than 41 decimal places, which
is a much greater precision and accuracy than that of even the most
well-determined physical constants. For comparison, the charge of the
electron is known to only seven decimal places, the Planck constant is
known to only eight decimal places, the mass of the neutron, proton, and
electron are all known to only nine decimal places, and the universal
gravitational constant has been determined to only three decimal places.
Transitional Forms and the Tree of Life
Any fossilized animals found should conform to the standard
phylogenetic tree. If all organisms are united by descent from a common
ancestor, then there is one single true historical phylogeny for all
organisms, just like there is one single true historical genealogy for
any individual human. It directly follows that if there is a unique
universal phylogeny, then all organisms fit in that phylogeny uniquely.
We have found a quite complete set of dinosaur (reptile)-to-bird
transitional fossils with no morphological gaps, represented by Eoraptor,
Herrerasaurus, Ceratosaurus, Allosaurus, Compsognathus, Sinosauropteryx,
Protarchaeopteryx, Caudipteryx, Velociraptor, Sinovenator, Beipiaosaurus,
Sinornithosaurus, Microraptor, Archaeopteryx, Rahonavis, Confuciusornis,
Sinornis, Patagopteryx, Hesperornis, Apsaravis, Ichthyornis, and Columba,
among many others. We also have an exquisitely complete series of
fossils for the reptile-to-mammal intermediates, ranging from the
pelycosauria, therapsida, cynodonta, up to primitive mammalia.
Based upon the consensus of numerous phylogenetic analyses, Pan
troglodytes (the chimpanzee) is the closest living relative of humans.
Thus, we expect that organisms lived in the past which were intermediate
in morphology between humans and chimpanzees. Over the past century,
many spectacular paleontological finds have identified such transitional
hominid fossils. Another impressive example of incontrovertible
transitional forms predicted to exist by evolutionary biologists is the
collection of land mammal-to-whale fossil intermediates. Whales,
of course, are sea animals with flippers. Since they are also mammals,
the consensus phylogeny indicates that whales and dolphins evolved from
land mammals with legs. In recent years, we have found several
transitional forms of whales with legs, both capable and incapable of
terrestrial locomotion (for some pictures and more details, see "Transitional Fossils"
below).
The reptile-bird intermediates
date from the Upper Jurassic and Lower Cretaceous (about 150
million years ago), whereas pelycosauria and therapsida (reptile-mammal
intermediates) are older and date from the Carboniferous and the
Permian (about 250 to 350 million years ago, see the Geological
Time Scale at right). This is precisely what should be observed if
the fossil record matches the standard phylogenetic tree. The
most scientifically rigorous method of confirming this is to
demonstrate a positive correlation between phylogeny and
stratigraphy (the strata or rocks of the geologic column where
fossils are found
throughout the world), i.e. a positive correlation between the
order of taxa in a phylogenetic tree and the geological order in
which those taxa first appear and last appear (whether for
living or extinct intermediates).
The Geological Time Scale and Fossil Record
The geological periods where the major groups of organisms appeared
can be divided as follows (see also Kenneth Miller, Finding
Darwin's God, page 39) : Ma = Millions of years ago
CENOZOIC
Quaternary 1.5-present Ma -- modern humans appear (Homo sapiens
sapiens)
Tertiary 65-1.5 Ma -- Mammals and birds and teleost fish dominant
 MESOZOIC
MESOZOIC
Cretaceous 144-65 Ma -- Dinosaurs dominant, small mammals, birds
Jurassic 213-144 Ma -- Dinosaurs dominant, first mammals, then first
birds
Triassic 248-213 Ma -- Mammalian reptiles dominant, first dinosaurs
PALEOZOIC
Permian 286-248 Ma -- Amphibians dominant, first mammal-like reptiles
Pennsylvanian 320-286 Ma -- Amphibians dominant, first reptiles
Carboniferous (includes Penn and Miss periods)
Mississippian 360-320 Ma -- big terrestrial amphibians, fishes
Devonian 408-360 Ma -- Fish dominant, first amphibians
Silurian 438-408 Ma -- first ray-finned and lobe-finned fish
Ordovician 505-438 Ma -- more jawless fishes
Cambrian 570-505 Ma -- first jawless fishes
Within the error inherent in the fossil record, prokaryotes should
appear first, followed by simple multicellular animals like sponges and
starfish, then lampreys, fish, amphibians, reptiles, then mammals, etc.
Studies from the past ten years addressing this very issue have
confirmed that there is indeed a positive correlation between phylogeny
and stratigraphy, with statistical significance. Using three different
measures of phylogeny-stratigraphy correlation [the RCI, GER, and SCI],
a high positive correlation was found between the standard phylogenetic
tree and the stratigraphic range of the same taxa,
with very high statistical significance (P < 0.0001).
It would be highly inconsistent if the chronological order were
reversed in the reptile-bird and reptile-mammal example.
More generally, the strongest falsification of this prediction would be
the finding that there was a negative correlation between stratigraphy
and the phylogenetic tree that describes the genealogical relatedness of
all living organisms.
History: Anatomical Vestiges and Atavisms
Some of the more renowned evidences for evolution are the various
nonfunctional or rudimentary vestigial characters, both anatomical and
molecular, which are found throughout biology. During macroevolutionary
history, functions necessarily have been gained and lost. Thus, from
common descent and the constraint of gradualism, we predict that many
organisms should display vestigial structures, which are structural
remnants of lost functions.
There are many examples of rudimentary and nonfunctional characters
carried by organisms, and these can very often be explained in terms of
evolutionary histories: (1) snakes such as pythons (which are
legless snakes) carry vestigial pelvises hidden beneath their skin; (2)
some lizards carry rudimentary, nonfunctional legs underneath
their skin, undetectable from the outside; (3) many cave dwelling
animals, such as the fish Astyanax mexicanus (the Mexican tetra) and
the salamander species Typhlotriton spelaeus and Proteus anguinus, are
blind yet have rudimentary, vestigial eyes; (4) dandelions
reproduce without reproduction (a condition known as apomixis), yet they
retain flowers and produce pollen (which are useless); (5) over 90% of
all adult humans develop third molars (otherwise known as wisdom
teeth), and in one-third they are malformed and impacted (these useless
structures point to our ancestors who were herbivorous, and molar teeth
were required for chewing and grinding plant material, but now in human
beings can cause significant pain and increased risk for injury, etc);
(6) there are many examples of flightless beetles (such as
the weevils of the genus Lucanidae) which retain perfectly formed wings
housed underneath fused wing covers. All of these examples can be
explained in terms of the beneficial functions and structures of the
organisms' predicted ancestors.
Anatomical atavisms are closely related conceptually to vestigial
structures. An atavism is the reappearance of a lost character specific
to a remote evolutionary ancestor and not observed in the parents or
recent ancestors of the organism displaying the atavistic character. As
with vestigial structures, no organism can have an atavistic structure
that was not previously found in one of its ancestors.
Probably the most well known case of atavism is found in the whales.
According to the standard phylogenetic tree, whales are known to be the
descendants of terrestrial mammals that had hindlimbs. Thus, we expect
the possibility that rare mutant whales might occasionally develop
atavistic hindlimbs. In fact, there are many cases where whales have
been found with rudimentary atavistic hindlimbs in the wild: hindlimbs have been found in baleen whales, humpback whales, and in many
specimens of sperm whales. Most of these examples are of whales with
femurs, tibia, and fibulae; however, some even include feet with
complete digits.
Other famous examples of atavisms exist, including (1) rare formation
of extra toes in horses (2nd and 4th digits), similar to what is
seen in the archaic horses Mesohippus and Merychippus; (2) atavistic thigh
muscles in birds and sparrows (Passeriform); (3) hyoid muscles in
dogs; (4) wings in earwigs (normally wingless); (5) atavistic
fibulae in birds (the fibulae are normally extremely reduced);
(6) extra toes in guinea pigs and salamanders; (6) the atavistic dew
claw in many dogs; and (7) various atavisms in humans -- such as the "true
human tail." Concerning the latter, more than 100 cases of
human tails have been reported in the medical literature and less than
one-third of these are medically known as "pseudo-tails"
(which are not true tails). True human tails are complex structures
which have muscle, blood vessels, occasional vertebrae and cartilage,
can move and contract, and they are occasionally inherited.
Vestigial characters are also found at the molecular level: (1) the
L-gulano-g-lactone oxidase gene, the gene required for Vitamin C
synthesis, was found in humans and guinea pigs, and in other primates
(chimpanzees, orangutans, and macaques), exactly as predicted by
evolutionary theory (it exists as a pseudogene, present but incapable of
functioning); (2) multiple odorant receptor genes; (3) the RT6 protein
gene; (4) the galactosyl transferase gene; and (5) the tyrosinase-related
gene (TYRL). We share these vestigial genes with other primates, and the mutations that made these genes nonfunctional are also shared
with several other primates.
Embryology
Embryology and developmental biology have provided some fascinating
insights into evolutionary pathways. Since the cladistic morphological
classification of species is generally based on derived characters of
adult organisms, embryology and developmental studies provide a nearly
independent body of evidence. From embryological studies it is known
that two bones of a developing reptile eventually form the quadrate and
the articular bones in the hinge of the adult reptilian jaw.
Accordingly, there is a very complete series of fossil intermediates in
which these structures are clearly modified from the reptilian jaw to
the mammalian ear.
Early in development, mammalian embryos temporarily have pharyngeal
pouches, which are morphologically indistinguishable from aquatic
vertebrate gill pouches. This evolutionary relic reflects the fact that
mammalian ancestors were once aquatic gill-breathing vertebrates. The
arches between the gills, called branchial arches, were present in
jawless fish and some of these branchial arches later evolved into the
bones of the jaw, and, eventually, into the bones of the inner ear.
Many species of snakes and legless lizards (such as the "slow
worm") initially develop limb buds in their embryonic development,
only to reabsorb them before hatching. Similarly, modern adult whales,
dolphins, and porpoises have no hind legs. Even so, hind legs, complete
with various leg bones, nerves, and blood vessels, temporarily appear in
the cetacean fetus and subsequently degenerate before birth.
Mammals evolved from a reptile-like ancestor, and placental mammals
(like humans and dogs) have lost the egg-tooth and caruncle (and
eggshell). However, monotremes, such as the platypus and echidna, are
primitive mammals that have both an egg-tooth and a caruncle, even
though the monotreme eggshell is thin and leathery. Most strikingly,
during marsupial development, an eggshell forms transiently and then is
reabsorbed before live birth. Though they have no need for it, several
marsupial newborns (such as baby Brushtail possums, koalas, and
bandicoots) retain a vestigial caruncle as a clear indicator of their
reptilian, oviparous ancestry.
The fossil record has confirmed that birds once had teeth, as
demonstrated by the fossils of many birds with teeth including
Archaeopteryx. Furthermore, this predicted possibility has been
confirmed experimentally in a modern bird, the chicken. Kollar and
Fisher transplanted a small piece of mammalian mesenchymal tissue (which
forms teeth) underneath the beak-forming epithelial layer of a
developing chick. Intriguingly, they observed that the chicken
epithelium secreted dental enamel and directed the adjacent mesenchyme
to form teeth. This would have been impossible unless the chicken still
retained the genes and developmental pathway for making teeth. Thus,
chickens have not yet completely lost the genes coding for tooth
development (two of Stephen Jay Gould's popular books are titled Hen's
Teeth and Horse's Toes and The Panda's Thumb which
explain some of this past evolutionary history).
Present and Past Biogeography
Common ancestors originate in a particular geographical location.
Thus, the spatial and geographical distribution of species should be
consistent with their predicted genealogical relationships. The standard
phylogenetic tree predicts that new species must originate close to the
older species from which they are derived. Closely related contemporary
species should be close geographically, regardless of their habitat or
specific adaptations (if not, there should be a good explanation, such
as extreme mobility in the case of birds, sea animals, or human
intervention).
Examples of present biogeography supporting evolutionary theory are
(1) marsupials (kangaroos, etc) which only inhabit Australia
(exceptions such as some South American species and the opossum are
explained by continental drift); (2) conversely, placental mammals
are virtually absent on Australia, despite the fact that many would
flourish there (humans introduced most of the few placentals found on
Australia); (3) the southern reaches of South America and Africa
and all of Australia share lungfishes, ostrich-like birds (ratite
birds), and leptodactylid frogs -- all of which occur nowhere else;
(4) alligators, some related species of giant salamander,
and magnolias only occur in Eastern North America and East Asia (which
were once spatially close in the Laurasian continent); (5) indigenous Cacti
(Cactus plant) only inhabit the Americas, while Saharan and Australian
vegetation is very distantly related (mostly Euphorbiaceae); (6) members
of the closely related pineapple family inhabit many diverse
habitats (such as rainforest, alpine, and desert areas), but only in the
American tropics, not African or Asian tropics, etc.
As for past biogeography, we find the earliest marsupial fossils
(e.g. Alphadon) from the Late Cretaceous, when South America,
Antarctica, and Australia were still connected. Additionally, the
earliest ancestors of modern marsupials are actually found on North
America. The obvious paleontological deduction is that extinct marsupial
fossil organisms should be found on South America and Antarctica, since
marsupials must have traversed these continents to reach their present
day location in Australia. Interestingly, we have found marsupial
fossils on both South America and on Antarctica. This is an astounding
macroevolutionary confirmation, given that no marsupials live on
Antarctica now.
The Equidae (i.e. horse) fossil record is very complete
(though extremely complex) and makes very good geographical sense,
without any large spatial jumps between intermediates. Every single one
of the fossil ancestors of the modern horse are found on the North
American continent. Finally, the theory of common descent predicts that
we may find early hominid fossils on the African continent.
Numerous transitional fossils between humans and the great apes have
been found in southern and eastern Africa. Examples include Ardipithecus
ramidus, Australopithecus anamensis, Australopithecus afarensis,
Australopithecus garhi, Kenyanthropus platyops, Kenyanthropus
rudolfensis, Homo habilis, and a host of other transitionals thought to
be less related to Homo sapiens, such as the robust australopithicenes.
The Opportunistic Nature of Evolution and Evolutionary Constraint
The principle of evolutionary opportunism is closely related to
evolutionary history and to the effects of contingency. Descent with
gradual modification means that new organisms can only use and modify
what they initially are given; they are slaves to their history. New
structures and functions must be recruited from previous, older
structures. One major consequence of the constraint of gradualism is the
predicted existence of "paralogy": similarity of structure
despite difference in function.
Anatomical and Molecular Paralogy
There are countless examples of paralogy in living and extinct
species -- the same bones in the same relative positions are used in
primate hands, bat wings, bird wings, pterosaur wings, whale and penguin
flippers, horse legs, the digging forelimbs of moles, and webbed
amphibian legs. All of these characters have similar structures that
perform various different functions. The standard phylogenetic tree
shows why these species have these same structures, i.e. they have
common ancestors that had these structures. The fossil record shows a
general chronological progression of intermediate forms between theropod
dinosaurs and modern birds, in which theropod structures were modified
into modern bird structures.
On the molecular level, the existence of paralogy is quite
impressive. Many proteins of very different function have strikingly
similar amino acid sequences and three-dimensional structures. A
frequently cited example is lysozyme and a-lactalbumin. A-Lactalbumin is
very similar structurally to lysozyme, even though its function is very
different (it is involved in mammalian lactose synthesis in the mammary
gland). On a grander scale, a stunning confirmation of these
evolutionary predictions has come from an analysis of Saccharomyces
cerevisiae (baker's yeast) and Caenorhabditis elegans (a worm). The
genes used by the yeast, a unicellular organism, are mostly genes
dealing directly with core biochemical functions that all organisms must
perform. From an evolutionary perspective, we would expect these genes
to be ancient. Thus it was expected and shown that the worm contains a
great majority of these genes. In contrast, the extra genes used by the
worm, which deal with multicellularity, should be more recently evolved.
Phylogenetic analysis has shown that this is exactly the case. An even
larger study of the known eukaryotic genomes has further demonstrated
that paralogy is rampant in nature, and that true structural innovation
is relatively rare ("Comparative Genomics of the Eukaryotes"
[2000] Science
287: 2204-2218).
Anatomical and Molecular Analogy
A corollary of the principle of evolutionary opportunism is analogy.
Analogy is the case where different structures perform the same or
similar functions in different species. Two distinct species have
different histories and different structures; if both species evolve the
same new function, they may recruit different structures to perform this
new function. Analogy also must conform to the principle of structural
continuity; analogy must be explained in terms of the structures of
predicted ancestors. There are many anatomical examples of functional
analogy. One case is the vertebrate eye and the cephalopod eye. Another
is the case of American and Saharan desert plants, which use different
structures for the same functions needed to live in dry, arid regions.
By contrast, we would not expect newly discovered species of dolphins,
whales, penguins, or any close mammalian relatives to have gills (a
possible analogy with fish), since their immediate ancestors lacked
gills or gill-like structures from which they could be derived.
A familiar molecular example is the case of the three proteases
subtilisin, carboxy peptidase II, and chymotrypsin. These three proteins
are all serine proteases (i.e. they degrade other proteins in
digestion). They have the same function, the same catalytic residues in
their active sites, and they have the same catalytic mechanism. Yet they
have no sequence or structural similarity. Another molecular example is
that of DNA polymerases. Rat polymerase � has obviously evolved from
nucleotidyl transferases by mutating to catalyze several nucleotide
additions instead of just one -- which nicely illustrates why analogy is
ultimately also paralogy.
Suboptimal Function
Another consequence of evolutionary opportunism is the existence of
apparent suboptimal function. This does not refer to a structure
functioning poorly. It simply means that a structure with a more
efficient design (usually with less superfluous complexity), could
perform the same final function equally as well. Structures with
suboptimal function should have a gradualistic historical evolutionary
explanation, based on the opportunistic recruitment of ancestral
structures.
For example, the mammalian gastrointestinal tract crosses the
respiratory system. Functionally, this is suboptimal; it would be
beneficial if we could breathe and swallow simultaneously. However,
there is a good historical evolutionary reason for this arrangement. The
Osteolepiformes (Devonian lungfish), from which mammals evolved,
swallowed air to breathe. Only later did the ancestors of mammals
recruit the olfactory nares of fish for the function of breathing on
land. Another anatomical example of suboptimal function is the inverted
mammalian retina, with its blind spot. In order to deal with the many
problems inherent in an inverted retina, the vertebrate eye utilizes
various complex compensatory structures and mechanisms. In contrast with
mammalian eyes, cephalopod eyes have very different underlying retinal
structures (e.g. they are verted, not inverted), and they have no blind
spots. This strongly suggests that mammals also could have eyes without
blind spots.
With the recent sequencing of the human genome, we have found that
less than 2% of the DNA in the human genome is used for making proteins
(International Human Genome Sequencing Consortium 2001). A full 45% of
our genome is composed of transposons, which serve no known function for
the individual (except to cause a significant fraction of genetic
illnesses and cancers). Twenty percent of the human genome are
pseudogenes. They also serve no function for the individual. A
remarkable example is the glyceraldehyde-3-phosphate dehydrogenase (GDPH)
gene. In humans, there is one functional GDPH gene, but there are at
least twenty GDPH pseudogenes. In mice, there are approximately 200 GDPH
pseudogenes, none of which are necessary. In addition to one or two
functional copies, there are between 20 and 30 pseudogenes of cytochrome
c in both humans and the rat.
A lot of wasted energy is expended in dealing with this useless DNA;
however, all these molecular examples also have convincing explanations
based on evolutionary histories.
The Molecular Sequence Evidence
The molecular sequence evidence gives the most impressive and
irrefutable evidence for the genealogical relatedness of all life. The
nature of molecular sequences allows for extremely impressive
probability calculations that demonstrate how well the predictions of
common descent with modification actually match empirical observation.
There are several categories and independent lines of molecular sequence
evidence useful for determining phylogenetic relationships. Studies of functional
elements include ribosomal RNA, ubiquitous proteins, and mitochondrial
DNA comparisons; studies of nonfunctional elements include
comparisons of pseudogenes, endogenous retroviral genes, and mobile
genetic elements (such as introns, transposons, or retroelements).
Cytochrome c Studies
Cytochrome c is an essential and ubiquitous protein found in
all organisms, including eukaryotes and bacteria. The mitochondria of
cells contain cytochrome c, where it transports electrons in the
fundamental metabolic process of oxidative phosphorylation. The oxygen
we breathe is used to generate energy in this process. Using an
ubiquitous gene such as cytochrome c, there is no reason to assume that
two different organisms should have the same protein sequence or even
similar protein sequences, unless the two organisms are genealogically
related. Hubert Yockey has done a careful study in which he calculated
that there are a minimum of 2.3 x 1093 possible functional
cytochrome c protein sequences, based on genetic mutational
analyses (Yockey, H.P. [Cambridge Univ Press, 1992] Information
Theory and Molecular Biology, Chapter 6). For perspective, the
number 1093 is about one billion times larger than the number
of atoms in the visible universe. Thus, functional cytochrome c
sequences are virtually unlimited in number, and there is no a priori
reason for two different species to have the same, or even mildly
similar, cytochrome c protein sequences.
From the theory of common descent and our standard phylogenetic tree
we know that humans and chimpanzees are quite closely related. We
therefore predict, in spite of the odds, that human and chimpanzee
cytochrome c sequences should be much more similar than, say, human and
yeast cytochrome c -- simply due to inheritance. This has been confirmed: Humans and chimpanzees have the exact same
cytochrome c protein sequence. In the absence of common descent, the
chance of this occurrence is conservatively less than 10-93 (1
out of 1093). Thus, the high degree of similarity in these
proteins is a spectacular corroboration of the theory of common descent.
Furthermore, human and chimpanzee cytochrome c proteins differ by about
10 amino acids from all other mammals. The chance of this occurring in
the absence of a hereditary mechanism is less than 10-29.
Further, bat cytochrome c is much more similar to human cytochrome c
than to hummingbird cytochrome c; porpoise cytochrome c is much more
similar to human cytochrome c than to shark cytochrome c. The
phylogenetic tree constructed from the cytochrome c data exactly
recapitulates the relationships of major taxa as determined by
the completely independent morphological data. Why would two organisms
have such similar ubiquitous proteins when the odds are astronomically
against it? We know of only one reason for why two organisms would have
two similar protein sequences in the absence of functional necessity:
heredity. Thus, in such cases we can confidently deduce that the two
organisms are genealogically related.
Like protein sequence similarity, the DNA sequence similarity of two
ubiquitous genes also implies common ancestry. If chimps and humans are
truly genealogically related, we predict that the difference between
their respective cytochrome c gene DNA sequences should be less than 3%
-- probably even much less, due to the essential function of the
cytochrome c gene. As mentioned above, the cytochrome c proteins in
chimps and humans are exactly identical. The clincher is
that the two DNA sequences that code for cytochrome c in humans and
chimps differ by only one base (a 0.3% difference), even
though there are 1049 different sequences that could code for
this protein. The combined effects of DNA coding redundancy and protein
sequence redundancy make DNA sequence comparisons doubly redundant; DNA
sequences of ubiquitous proteins are completely uncorrelated with
phenotype, but they are strongly causally correlated with heredity. This
is why DNA sequence phylogenies are considered so robust.
Pseudogenes
Other nonfunctional molecular examples that provide evidence of
common ancestry are pseudogenes. Pseudogenes are very closely related to
their functional counterparts (in primary sequence and often in
chromosomal location), except that either they have faulty regulatory
sequences or they have internal stops that keep the protein from being
made. They are functionless and do not affect an organism's phenotype
when deleted. Finding the same pseudogene in the same chromosomal
location in two species is strong evidence of common ancestry.
This also has been confirmed: there are very many examples of shared
pseudogenes between primates and humans. One is the ψη-globin
gene, a hemoglobin pseudogene. It is shared among the primates only, in
the exact chromosomal location, with the same mutations that render it
nonfunctional. Another example is the steroid 21-hydroxylase gene.
Humans have two copies of the steroid 21-hydroxylase gene, a functional
one and a nonfunctional pseudogene. Chimps and humans both share the same
eight bp deletion in this pseudogene that renders it
nonfunctional.
Conclusion
These previous points are all evidence of macroevolution alone; the
evidence and the conclusion are independent of any specific gradualistic
explanatory mechanisms for the origin and evolution of macroevolutionary
adaptations and variation. This is why scientists call universal common
descent the "fact of evolution." None of the evidence above
assumes that natural selection is true or that it is sufficient for
generating adaptations or the differences between species and other taxa.
Thus, the macroevolutionary conclusion stands, regardless of the
mechanism.
Adapted from the detailed TalkOrigins "29+ Evidences for
Macroevolution" (from November 2002 version) by Douglas Theobald, Ph.D.
Transitional Fossils
(being updated)
First, some Catholic writers on the false "No Transitional
Fossils" claim:
KARL KEATING of Catholic Answers:
"The writer quoted prominent biologists -- evolutionists to a man -- who affirm that the missing links are still missing. The fossil record, they say, fails to show even one clear example of species A turning into species B. It shows many examples of one species disappearing and being replaced by another, but that is not the same thing. It also shows the development of minor variations within species but never a transition from one species into another. As one might expect, this is awkward for Darwin's theory, which holds that species develop from one another through a long series of minute changes. The quoted biologists do not reject evolution itself, but they say that the scheme given in the Origin of Species is not supported by the fossil record....I am not a biologist, and I do not have sufficient interest in the question of speciation to work up a knowledgeable conclusion on my own. But what does interest me is that people who are biologists and who do have demonstrated sufficient interest have come to opposite conclusions. One group says the links are missing, and the other says they have been found. It is not possible that both groups are correct, since the same link cannot be both missing and found." (Karl Keating,
"At Ease Please," This Rock magazine, July-Aug 2006)
ROBIN BERNHOFT of Kolbe Center for Creation:
"The fossil record is equally hostile to Darwin....He expected that many fossils would be found of species intermediate between ancestral organisms and their descendants and admitted that if such fossils could not be found it would disprove his theory. By Darwin's own criterion his theory has been disproved. In the past one hundred fifty years, the fossil record has become nearly complete, yet there are still no intermediate fossils. Scientists have found fossils of 97.7 percent of land vertebrates worldwide, and almost one hundred percent in North America, and still they have not found the intermediate fossils Darwin said had to be there in order for his theory to be true....What you find in the fossil record is the sudden appearance, 600 million years ago in the 'Cambrian Explosion,' of a wide range of mature fossils. Some of these lasted for a while, then died out. Others have survived into the present. None changed into anything else. Later, other fossils appeared abruptly in their mature forms, persisted, then either died out or survived to the present. None changed into anything else. There are no intermediate forms....The fossil record provides no evidence that any species was ancestral to any other species and no evidence of intermediate forms showing ancestral relationship.....Finally, there is no scientific evidence that microevolution -- the adaptation of species to environmental change -- can generate macroevolution -- the development of new species." (Robin
Bernhoft, "Confronting Creation's Complexities: Darwinism Isn't Fit to Survive,"
This Rock magazine, Sept 2003)
GERARD KEANE of Kolbe Center for Creation:
"In reality, not only are the
required intermediate forms between the various species absent from the
fossil record, but also many such supposed forms are conceptually
untenable. Evolution Theory now stands exposed as both the worst mistake
made in science and the most enduring myth of modern times....If
Evolution really did occur down through the ages, an ample number of
transitional creatures should by now have been found among the immense
number of fossils now unearthed....Vast numbers of fossils identical to
creatures alive today have been found, but not a trace of transitional
forms. The fossil record is devoid of 'missing links' grading up from
simple to more complex creatures." (Gerard Keane, Creation
Rediscovered [Tan Books, 1999], pages xxvi, 103, 105)
AMY WELBORN, blogger and author of "Prove It" series of
books, she outlines three "basic problems" with evolution, the first one is:
"While 'microevolution' the development or disappearance of traits within a species is an acknowledged fact, the broader
Darwinian scheme 'macroevolution' which proposes that the diversity of living creatures comes from one organism adapting so much it became a whole
other species, suffers from a serious lack of evidence at this point. There is absolutely no evidence from the fossil record of evolution between species. No 'transitional' or 'intermediate' forms exist. The missing link is still missing." (Amy
Welborn, Prove It! God [2000], page 46)
GEORGE SIM JOHNSTON, author of Did Darwin Get It Right?
"The publication of the Origin sent whole armies of paleontologists into the folds of the earth to find the 'innumerable' transitional links that Darwin said must be there. What did this army find? The answer appears to be -- nothing. The fossil evidence does not support the idea that species evolved by minute gradations....The fossil record shows exactly what it shows in Darwin's day -- that species appear suddenly in a fully developed state and change little or not at all before disappearing...." (George Sim Johnston,
Did Darwin Get It Right? Catholics and the Theory of Evolution
[1998], page 29-30)
"...about 550 million years ago, came biology's Big Bang. It occurred at the beginning of the Cambrian era. There was an explosion of highly organized life-forms -- mollusks, jellyfish, trilobites -- for which not a single ancestral fossil can be found in the earlier rocks....The Precambrian strata, moreover, are perfectly suited for the imprinting of fossils. In some locations, there are over five thousand feet of unbroken layers of sedimentary rock; but they do not contain the innumerable transitional species that Darwin maintained to be there. They are, in fact, an evolutionary blank....More importantly, there are no transitional forms, no gradations to speak of, leading up to these complex animals....This compacting of the Cambrian explosion [he places it at only 5 million years] is a major problem for orthodox Darwinists." (Johnston, page 30, 32)
"In recent years paleontologists have retreated from simple connect-the-dot scenarios linking earlier and later species. Instead of ladders, they now talk of bushes. What we see in the fossils, according to this view, are only the twigs, the end products of evolution, while the key transitional forms that would give a clue about the origin of major animal and plant groups remain hidden. The gaps on the evolutionary trees occur at just the points where the crucial changes had to take place. The direct ancestors of all the major groups -- reptiles, mammals, flowering plants -- are missing. There are no fossil grandparent of the monkeys, for example....[quotes Don Johanson]....The same is true of bats, elephants, and turtles: They all simply burst upon the scene --
de novo, as it were." (Johnston, page 35)
FR. MITCH PACWA on "Mother Angelica Live" (Nov 1996)
"Darwin's theory has also been rejected by scientists. And that, one of the things that is important about that, you know, Darwin said there would be all kinds of missing links, from one species to another. Never have any missing links been found. The theory didn't work. The hypothesis failed."
(Fr. Mitch Pacwa, "Mother Angelica Live" Nov 1996 -- LISTEN
TO THIS)
Next, we are about to see how wrong these Catholics are on the
science. It's always best to read the actual paleontological literature,
rather than "quotes" from other creationists.
The Fossil Evidence for
Evolution
 The following data is adapted from the detailed TalkOrigins "Transitional
Vertebrate Fossils FAQ" (1994-1997) by Kathleen Hunt, and
updated from the book Evolution: What the Fossils Say and Why It
Matters by paleontologist / geologist Donald R. Prothero
(Columbia Univ Press, 2007). The following data is adapted from the detailed TalkOrigins "Transitional
Vertebrate Fossils FAQ" (1994-1997) by Kathleen Hunt, and
updated from the book Evolution: What the Fossils Say and Why It
Matters by paleontologist / geologist Donald R. Prothero
(Columbia Univ Press, 2007).
Abbreviations used: Ma = millions of years ago (Ga = billion), or My = millions of
years, where appropriate.
Please consult the
following books for the full paleontological evidence, pictures and
descriptions
of transitionals (also the Bibliography at end):
-
Vertebrate Paleontology and Evolution by Robert L. Carroll (1988) --
older source and I have double-checked the references made to
it
-
Evolution: What the Fossils Say and Why It Matters by
Donald R. Prothero (2007) -- updates Carroll and responds to many bad
"creationist" arguments and errors
- also Invertebrate Paleontology and Evolution by
E.N.K.
Clarkson (1979, 1998 4th edition) -- discusses the pre-Cambrian to
Cambrian fossils
- also
The Fossil Record 2 edited by M.J. Benton (1993) -- over
800 large pages that lists virtually all the known fossils from the animal
invertebrates, animal vertebrates, plants and other organisms
Carroll begins his comprehensive 1988 book on vertebrate
paleontology, evolution and the fossil record:
"During the past 20 years [i.e.
1968-1988], our knowledge of fossil vertebrates has
increased immensely. Entirely new groups of jawless fish, sharks,
amphibians, and dinosaurs have been discovered, and the major
transitions between amphibians and reptiles, reptiles and mammals, and
dinosaurs and birds have been thoroughly studied. Evidence from both
paleontology and molecular biology provides much new information on the
initial radiation of both birds and placental mammals." (Carroll, page
xiii preface).
Prothero updates us on the fossil record and evolution in the 20
years since Carroll's book:
"But the past 20 years [i.e.
1987-2007] have produced some of the greatest discoveries of all,
including incredible fossils that show how whales, manatees, and seals
evolved from land mammals, where elephants, horses, and rhinos come
from, and how the first backboned animals evolved. We now have an
amazing diversity of fossil humans, including specimens that show that
we walked upright on two feet almost 7 million years ago, long before
we acquired large brains. In addition to all this fossil evidence, we
have new evidence from molecules as well that enables us to decipher
the details of the family tree of life as never before....The fossil
record is an amazing testimony to the power of evolution, with
documentation of evolutionary transitions that Darwin could have only
dreamed about....The fossil record is now one of the strongest lines
of evidence for evolution, completely reversing its subordinate status
only 150 years ago. Instead of the embarrassingly poor record that
Darwin faced in 1859, we now have an embarrassment of riches." (Prothero
[2007], page xix-xx)
A couple of definitions and distinctions to keep in mind (from
Prothero, page 82ff, 124ff):
- while intermediate forms and transitional fossils between
species are rare, there are many transitionals between larger
groups (i.e. Classes such as Fish to Tetrapods, Reptiles to
Mammals, etc);
- an important concept is the distinction between a lineal
ancestor (i.e. direct ancestors such as father and
mother, grandparents, great-grandparents, etc) and a collateral
ancestor (uncles and aunts, great uncles/aunts, cousins or
"close relatives," etc), e.g. Archaeopteryx (front
cover of Prothero) has many transitional features between living
birds and Mesozoic dinosaurs, so if it was not direct it was
certainly a collateral ancestor;
- there is really no such thing as a "missing link" :
evolution is not about life climbing the "ladder of
nature" or a "great chain of being" from
"lower" to "higher" organisms; instead evolution
is more like a "bush" with many lineages branching from
one another, with "ancestors" living alongside their
"descendants"; the classification of life forms a natural
bushy or tree-like pattern;
- we do not need to have every single transitional fossil connecting
several species to show evolutionary transformation; a sequence of
related species that are stable through time nevertheless forms an
evolutionary transformation series even though not every
transitional fossil has been preserved, e.g. the evolution of sand
dollars from sea urchins (Prothero, page 190), etc;
- creationists challenge "evolutionists" to present a
"perfect 10" transitional fossil : this shows a
misunderstanding of evolution and transitional forms since there is
no general conversion of all parts of a transitional form at the
same time; genetics does not produce a smooth gradation of all
features, but characteristics of an intermediate will be mixed, a
pattern of descendents called mosaic evolution (i.e. normally
"cousins" of an ancestor, not lineal or direct descendants
due to the "incompleteness" or spottiness of the fossil
record and the multiple splitting off of species);
- since evolution is a bush, not a ladder, organisms evolve
but they do not always "move up the ladder"; species may
retain primitive features for hundreds of millions of years, and not
every anatomical feature of an animal evolves at the same time; some
parts may be quite "advanced" while others retain their
"primitive" state; this is the idea of mosaic
evolution and this is what we want for a transitional fossil.
Now I will cover many of the known and confirmed "transitional fossils" --
including some species-to-species transitions --
with a few pictures where appropriate. First we begin with the
controversial "origin of life" --
The Origin of Life on Earth
text here
text
Transitions from Invertebrates
to Vertebrates (and the "Cambrian explosion")
text here
From the book On
the Origin of Phyla by James W. Valentine (Univ of Chicago
Press, 2004) :
"The title of this book, modeled
on that of the greatest biological work ever written, is in homage
to the greatest biologist who has ever lived. Darwin puzzled over
but could not cover the ground that is reviewed here, simply because
the relevant fossils, genes, and their molecules, and even the
body-plans of many of the phyla, were quite unknown in his day. Nevertheless,
the evidence from these many additional sources of data simply
confirm that Darwin was correct in his conclusions that all living
things have descended from a common ancestor and can be
placed within a tree of life, and that the principle process guiding
their descent has been natural selection. And he was correct in so
much more." (Valentine, On the Origin of Phyla, preface
page xxiii)
text here
Transitions from primitive fish to sharks, skates, rays
Picture below is an early shark from the
late Devonian, Cladoselache (from Carroll, page 65).

 Cladoselache (late Devonian) --
Magnificent early shark fossils Cladoselache (late Devonian) --
Magnificent early shark fossils-
Tristychius and similar hybodonts (early Mississippian) --
Primitive proto-sharks with broad-based fins
-
Ctenacanthus and similar ctenacanthids (late Devonian) --
Primitive, slow sharks with broad-based shark-like fins and spines
-
Paleospinax (early Jurassic) -- More advanced features such as
detached upper jaw, but retains primitive ctenacanthid features
such as two dorsal spines, primitive teeth
-
Spathobatis (late Jurassic) -- First proto-ray
-
Protospinax (late Jurassic) -- A very early shark/skate
Picture to the right is a modern reef shark, photo by Bob Whorton and
SharkTrust.org
Transitions from primitive fish to bony fish
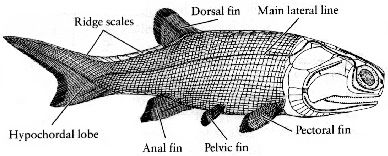
Picture below-right is the early Devonian Palaeoniscoid Moythomasia,
this genus shows the well-developed fulcral scales on the caudal fin
that are characteristic of chondrostean fishes (from Carroll, page 92).
 Acanthodians (Silurian) -- A
puzzling group of spiny fish with similarities to early bony fish Acanthodians (Silurian) -- A
puzzling group of spiny fish with similarities to early bony fish-
Palaeoniscoids, e.g. Cheirolepis, Mimia (early
Devonian) -- Primitive bony ray-finned fishes that gave rise to the vast
majority of living fish, heavy acanthodian-type scales, acanthodian-like
skull, and big notochord
-
Canobius, Aeduella (Carboniferous) -- Later paleoniscoids
with smaller, more advanced jaws
- Parasemionotus (early Triassic) -- "Holostean" fish
with modified cheeks but still many primitive features, almost exactly
intermediate between the late paleoniscoids and first teleosts, most of these fish lived in seasonal rivers and had lungs (which first
evolved in fish)
-
Oreochima and similar pholidophorids (late Triassic) -- The most
primitive teleosts, with lighter scales (almost cycloid), partially
ossified vertebrae, more advanced cheeks/jaws
-
Leptolepis and similar leptolepids (Jurassic) -- More advanced
with fully ossified vertebrae and cycloid scales, the Jurassic
leptolepids radiated into the modern teleosts (the massive, successful
group of fishes that are almost totally dominant today), lung
transformed into swim bladder
Transitions from fishes to first amphibians
(tetrapods)
- Paleoniscoids again, e.g.
Cheirolepis -- These ancient bony fish probably gave rise both to
modern ray-finned and lobe-finned fish
 Osteolepis (mid-Devonian) -- One of the earliest crossopterygian
lobe-finned fishes, sharing characters with the lungfish, had paired
fins with a leg-like arrangement of major limb bones, capable of flexing
at the "elbow" and had an early-amphibian-like skull and teeth Osteolepis (mid-Devonian) -- One of the earliest crossopterygian
lobe-finned fishes, sharing characters with the lungfish, had paired
fins with a leg-like arrangement of major limb bones, capable of flexing
at the "elbow" and had an early-amphibian-like skull and teeth-
Eusthenopteron, Sterropterygion (mid-late Devonian) --
Early rhipidistian lobe-finned fish roughly intermediate between early
crossopterygian fish and the earliest amphibians
-
Panderichthys, Elpistostege (mid-late Devonian, about 370
Ma) -- These "panderichthyids" are very tetrapod-like
lobe-finned fish, fragmented limbs and teeth from the mid-late Devonian
(about 370 Ma)
-
Obruchevichthys -- Discovered in 1991 in Scotland, some of the
earliest known tetrapod remains
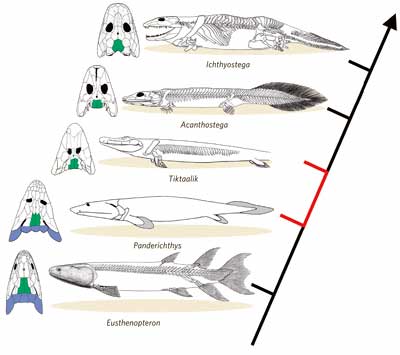
- Tiktaalik roseae ("large shallow water
fish") -- a 375-million-year-old large scaly creature with forward fins and the beginnings of digits, proto-wrists, elbows and shoulders, a flat skull like a crocodile,
a neck, ribs and other parts similar to tetrapods, discovered by a team led by Neil
Shubin of the University of Chicago in sediments of former streambeds in the Canadian Arctic, 600 miles from the North Pole
-
Hynerpeton, Acanthostega, Ichthyostega, Tulerpeton
(late
Devonian) -- A little later, the fin-to-foot transition was almost
complete, and we have a set of early tetrapod fossils that clearly did
have feet
-
Labyrinthodonts, e.g. Pholidogaster, Pteroplax (late
Dev/early Miss) -- These larger amphibians still have some icthyostegid
fish features, such as skull bone patterns, labyrinthine tooth dentine,
presence and pattern of large palatal tusks, the fish skull hinge,
pieces of gill structure between cheek and shoulder, and the vertebral
structure, but they have lost several other fish features: the fin rays
in the tail are gone, the vertebrae are stronger and interlocking, the
nasal passage for air intake is well defined

Picture above is the evolution of the earliest terrestrial tetrapods
(e.g. Acanthostega) from aquatic
lobe-finned fish (e.g. Eusthenopteron) that involved a transformation of the
skeleton. Among other changes, the pectoral and pelvic fins became limbs
with feet and toes, the vertebrae became interlocking, the tail fin
disappeared, the snout elongated, and the bones that covered the gills
and throat were lost. Many of Acanthostega's features were
undeniably fishlike, including both gills and lungs. [ Source: "Getting a Leg Up on Land" by
Jennifer Clack, Scientific American, December 2005 ]
 Transitions among amphibians Transitions among amphibians
- Temnospondyls, e.g. Pholidogaster
(Mississippian, about 330 Ma) -- A group of large labrinthodont
amphibians, transitional between the early amphibians and later
-
Archegosaurus decheni (early Permian) -- Intertemporals lost
-
Eryops megacephalus (late Penn) -- Occipital condyle splitting in
two
-
Trematops spp (late Permian) -- Eardrum like modern amphibians
-
Amphibamus lyelli (mid-Penn) -- Double occipital condyles, ribs
very small
-
Doleserpeton annectens or perhaps Schoenfelderpeton (both
early Permian) -- first pedicellate teeth (classic trait of modern
amphibians)
-
Triadobatrachus (early Triassic) -- a proto-frog, with a longer
trunk and much less specialized hipbone, and a short tail still present
-
Vieraella (early Jurassic) -- first known true frog
-
Karaurus (early Jurassic) -- first known salamander
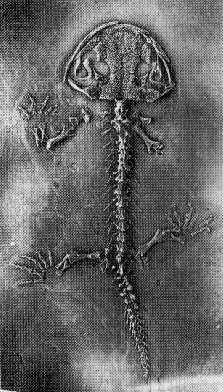
Picture to the right is the oldest known salamander, Karaurus,
from the early Jurassic of Russia. When they first appear in the fossil
record during the Jurassic, both frogs and salamanders appear
essentially modern in their skeletal anatomy (from Carroll, page
180-181).
Transitions from amphibians to first reptiles
- Proterogyrinus or another early
anthracosaur (late Mississippian) -- Classic labyrinthodont-amphibian
skull and teeth, but with reptilian vertebrae, pelvis, humerus, and
digits, still has fish skull hinge, amphibian ankle, 5-toed hand and a
2-3-4-5-3 (almost reptilian) phalangeal count
-
Limnoscelis, Tseajaia (late Carboniferous) -- Amphibians
apparently derived from the early anthracosaurs, but with additional
reptilian features: structure of braincase, reptilian jaw muscle,
expanded neural arches
-
Solenodonsaurus (mid-Pennsylvanian) -- An incomplete fossil,
apparently between the anthracosaurs and the cotylosaurs, loss of
palatal fangs, loss of lateral line on head, still just a single sacral
vertebra
-
Hylonomus, Paleothyris (early Pennsylvanian) -- These are
protorothyrids, very early cotylosaurs (primitive reptiles), quite little, lizard-sized animals with amphibian-like skulls (amphibian
pineal opening, dermal bone), shoulder, pelvis, limbs, intermediate
teeth and vertebrae, rest of skeleton reptilian, with reptilian jaw
muscle, no palatal fangs, and spool-shaped vertebral centra, probably no
eardrum yet
Many of these new "reptilian"
features are also seen in little amphibians (which also sometimes have
direct-developing eggs laid on land), so perhaps these features just
came along with the small body size of the first reptiles. The major
functional difference between the ancient, large amphibians and the
first little reptiles is the amniotic egg. Additional differences
include stronger legs and girdles, different vertebrae, and stronger jaw
muscles.
Transitions among reptiles (and dinosaurs)
- Scutosaurus and other pareiasaurs
(mid-Permian) -- Large bulky herbivorous reptiles with turtle-like skull
features
-
Deltavjatia vjatkensis (Permian) -- A recently discovered
pareiasaur with numerous turtle-like skull features
-
Proganochelys (late Triassic) -- a primitive turtle, with a fully
turtle-like skull, beak, and shell, but with some primitive traits such
as rows of little palatal teeth, a still-recognizable clavicle, a simple
captorhinid-type jaw musculature, a primitive captorhinid-type ear, a
non-retractable neck
-
Hylonomus, Paleothyris (early Penn) -- The primitive
amniotes described above Petrolacosaurus, Araeoscelis
(late Pennsylvanian), first known diapsids
-
Apsisaurus (early Permian) -- A more typical diapsid, lost
canines
-
Claudiosaurus (late Permian) -- An early diapsid with several
neodiapsid traits, but still had primitive cervical vertebrae and
unossified sternum, probably close to the ancestry of all diapsides (the
lizards, snakes, crocs, birds)
-
Planocephalosaurus (early Triassic) -- Further along the line
that produced the lizards and snakes, loss of some skull bones, teeth,
toe bones
-
Protorosaurus, Prolacerta (early Triassic) -- Possibly
among the very first archosaurs, the line that produced dinosaurs,
crocodiles,
and birds, may be "cousins" to the archosaurs
-
Proterosuchus (early Triassic), also Sphenosuchus -- First known
archosaurs/crocodiles
-
Hyperodapedon, Trilophosaurus (late Triassic) -- Early
archosaurs
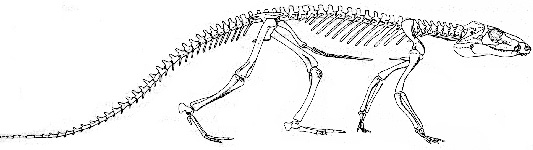 Picture
to the left is an early archosaur, Protosuchus, approx one meter
long, an early Triassic
Protosuchid crocodile. The Protosuchia retain long limbs and probably
were basically terrestrial in habit, but they resemble modern crocodiles
more closely than sphenosuchids in their general appearance and
configuration of the skull (from Carroll, page 280-281). Picture
to the left is an early archosaur, Protosuchus, approx one meter
long, an early Triassic
Protosuchid crocodile. The Protosuchia retain long limbs and probably
were basically terrestrial in habit, but they resemble modern crocodiles
more closely than sphenosuchids in their general appearance and
configuration of the skull (from Carroll, page 280-281).
Several possible cases of gradual
evolution (as well as some lineages that showed abrupt appearance or
stasis) among the early Permian reptile genera Captorhinus, Protocaptorhinus,
Eocaptorhinus, and Romeria are known. Excellent
transitional dinosaur fossils from a site in Montana that was a coastal
plain in the late Cretaceous include:
- Many transitional ceratopsids between Styracosaurus
and Pachyrhinosaurus
-
Many transitional lambeosaurids (50 specimens) between Lambeosaurus
and Hypacrosaurus
-
A transitional pachycephalosaurid between Stegoceras and Pachycephalosaurus
-
A transitional tyrannosaurid between Tyrannosaurus and Daspletosaurus
 All of these transitional animals lived
during the same brief 500,000 years. Before this site was studied, these
dinosaur groups were known from the much larger Judith River Formation,
where the fossils showed 5 million years of evolutionary stasis,
followed by the apparently abrupt appearance of the new forms. It turns
out that the sea level rose during that 500,000 years, temporarily
burying the Judith River Formation under water, and forcing the dinosaur
populations into smaller areas such as the site in Montana. While the
populations were isolated in this smaller area, they underwent rapid
evolution. When sea level fell again, the new forms spread out to the
re-exposed Judith River landscape, thus appearing "suddenly"
in the Judith River fossils, with the transitional fossils only existing
in the Montana site. All of these transitional animals lived
during the same brief 500,000 years. Before this site was studied, these
dinosaur groups were known from the much larger Judith River Formation,
where the fossils showed 5 million years of evolutionary stasis,
followed by the apparently abrupt appearance of the new forms. It turns
out that the sea level rose during that 500,000 years, temporarily
burying the Judith River Formation under water, and forcing the dinosaur
populations into smaller areas such as the site in Montana. While the
populations were isolated in this smaller area, they underwent rapid
evolution. When sea level fell again, the new forms spread out to the
re-exposed Judith River landscape, thus appearing "suddenly"
in the Judith River fossils, with the transitional fossils only existing
in the Montana site.
This is an excellent example of punctuated
equilibrium (500,000 years is very brief and counts as a
"punctuation"), and is a good example of why transitional
fossils may only exist in a small area, with the new species appearing
"suddenly" in other areas. Also note the discovery of Ianthosaurus,
a genus that links the two synapsid families Ophiacodontidae and Edaphosauridae.
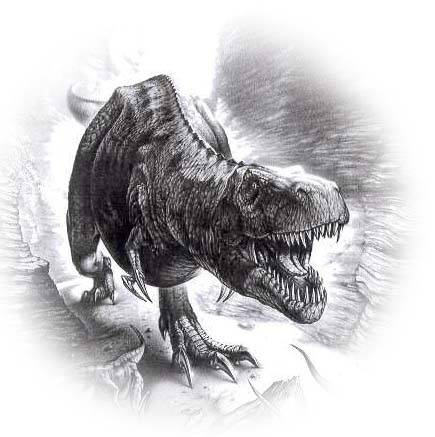
Picture above-left is the well-known Tyrannosaurus Rex
(artwork by Fabio Pastori), one of the largest meat-eating dinosaurs,
sharp teeth reaching lengths of 6 in, height up to 20 ft, length up to 49 ft, weight
approx 6.5 US tons, first complete T-rex skeleton was discovered in 1902, many great examples have been unearthed over the last several decades.
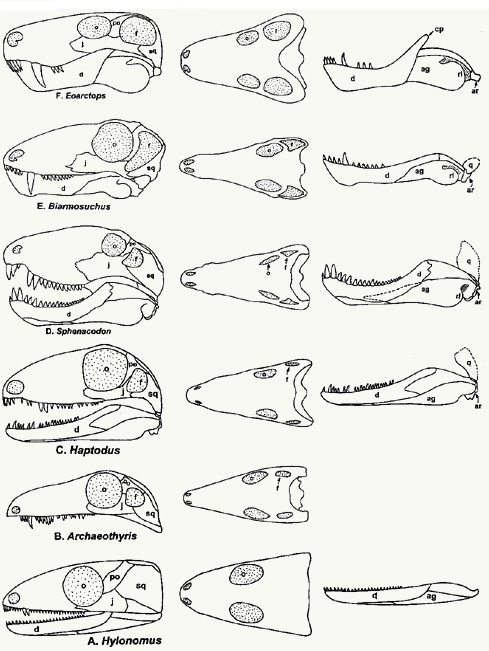 Transitions from reptiles to first mammals Transitions from reptiles to first mammals
This is the best-documented transition
between vertebrate classes. So far this series is known only as a series
of genera or families; the transitions from species to species are not
known. But the family sequence is quite complete. These range from the
pelycosauria, therapsida, cynodonta, up to primitive
mammalia.
- Paleothyris (early Pennsylvanian)
-- An early captorhinomorph reptile, with no temporal fenestrae at all
-
Protoclepsydrops haplous (early Pennsylvanian) -- The earliest
known synapsid reptile
-
Clepsydrops (early Pennsylvanian) -- The second earliest known
synapsid
-
Archaeothyris (early-mid Pennsylvanian) -- A slightly later
ophiacodont
-
Varanops (early Permian) -- Temporal fenestra further enlarged,
braincase floor shows first mammalian tendencies
-
Haptodus (late Pennsylvanian) -- One of the first known
sphenacodonts, showing the initiation of sphenacodont features while
retaining many primitive features of the ophiacodonts
-
Dimetrodon, Sphenacodon or a similar sphenacodont (late
Pennsylvanian to early Permian, 270 Ma) -- More advanced pelycosaurs,
clearly closely related to the first therapsids
-
Biarmosuchia (late Permian) -- A therocephalian, one of the
earliest, most primitive therapsids
-
Procynosuchus (latest Permian) -- The first known cynodont, a
famous group of very mammal-like therapsid reptiles, sometimes
considered to be the first mammals
-
Dvinia or Permocynodon (latest Permian) -- Another early
cynodont, first signs of teeth that are more than simple stabbing points
-
Thrinaxodon (early Triassic) -- A more advanced "galesaurid"
cynodont, further development of several of the cynodont features seen
already
-
Cynognathus (early Triassic, 240 Ma) -- Advanced cynodont, temporal fenestra larger, teeth differentiating further,
cheek teeth with cusps met in true occlusion for slicing up food
-
Diademodon (early Triassic, 240 Ma) -- Temporal fenestra larger
still, for still stronger jaw muscles, true bony secondary palate formed
exactly as in mammals, but didn't extend quite as far back
- Probelesodon (mid-Triassic; South America) -- Fenestra very
large, still separate from eyesocket (with postorbital bar)
 Probainognathus (mid-Triassic, 239-235 Ma, Argentina) -- Larger
brain with various skull changes: pineal foramen ("third eye")
closes, fusion of some skull plates Probainognathus (mid-Triassic, 239-235 Ma, Argentina) -- Larger
brain with various skull changes: pineal foramen ("third eye")
closes, fusion of some skull plates-
Exaeretodon (mid-late Triassic, 239 Ma, South America) -- Formerly
lumped with the herbivorous gomphodont cynodonts, mammalian jaw prong
forms, related to eardrum support, three incisors only (mammalian)
- Oligokyphus, Kayentatherium (early Jurassic, 208 Ma) --
These are tritylodontids, an advanced cynodont group, face more
mammalian, with changes around eyesocket and cheekbone, full bony
secondary palate
- Pachygenelus, Diarthrognathus (earliest Jurassic, 209 Ma)
-- These are trithelodontids, a slightly different advanced cynodont
group
-
Adelobasileus cromptoni (late Triassic, 225 Ma, west Texas) -- A
recently discovered fossil proto-mammal from right in the middle of that
late Triassic gap, currently the oldest known "mammal"
- Sinoconodon (early Jurassic, 208 Ma) -- The next known very
ancient proto-mammal
-
Kuehneotherium (early Jurassic, about 205 Ma) -- A slightly later
proto-mammal, sometimes considered the first known pantothere (primitive
placental-type mammal)
-
Eozostrodon, Morganucodon, Haldanodon (early
Jurassic, 205 Ma) -- A group of early proto-mammals called
morganucodonts, the restructuring of the secondary palate and the floor of the braincase
had continued, and was now very mammalian, truly mammalian teeth
-
Peramus (late Jurassic, about 155 Ma) -- A "eupantothere"
(more advanced placental-type mammal), the closest known relative of the
placentals and marsupials
- Endotherium (very latest Jurassic, 147 Ma) -- An advanced
eupantothere, fully tribosphenic molars with a well-developed talonid
-
Kielantherium and Aegialodon (early Cretaceous) -- More
advanced eupantotheres known only from teeth
-
Steropodon galmani (early Cretaceous) -- The first known definite
monotreme, discovered in 1985
-
Vincelestes neuquenianus (early Cretaceous, 135 Ma) -- A
probably-placental mammal with some marsupial traits, known from some
nice skulls
-
Pariadens kirklandi (late Cretaceous, about 95 Ma) -- The first
definite marsupial, known only from teeth
-
Kennalestes and Asioryctes (late Cretaceous, Mongolia) --
Small, slender animals, eyesocket open behind, simple ring to support
eardrum, primitive placental-type brain
-
Cimolestes, Procerberus, Gypsonictops (very late
Cretaceous) -- Primitive North American placentals with same basic tooth
pattern
Transitions from reptiles (dinosaurs) to first birds
In the mid-1800's, this was one of the
most significant gaps in vertebrate fossil evolution. No transitional
fossils at all were known, and the two groups seemed impossibly
different. Then the exciting discovery of Archaeopteryx in 1861
showed clearly that the two groups were in fact related. Since then,
other reptile-bird links have been found.
- Coelophysis (late Triassic) -- One
of the first theropod dinosaurs, theropods in general show clear general
skeletal affinities with birds (long limbs, hollow bones, foot with 3
toes in front and 1 reversed toe behind, long ilium)
-
Deinonychus, Oviraptor, and other advanced theropods (late
Jurassic, Cretaceous) -- Predatory bipedal advanced theropods, larger,
with more bird-like skeletal features
-
Lisboasaurus estesi and other "troodontid
dinosaur-birds" (mid-Jurassic) -- A bird-like theropod reptile with
very bird-like teeth (teeth very like those of early toothed birds,
since modern birds have no teeth)
-
Archaeopteryx lithographica (late Jurassic, 150 Ma) -- The
several known specimens of this deservedly famous fossil show a mosaic
of reptilian and avian features, with the reptilian features
predominating, the skull and skeleton are basically reptilian (skull,
teeth, vertebrae, sternum, ribs, pelvis, tail, digits, claws, generally
unfused bones)
-
Sinornis santensis ("Chinese bird", early Cretaceous,
138 Ma) -- A recently found little primitive bird with bird traits: short
trunk, claws on the toes, flight-specialized shoulders, stronger
flight-feather bones, tightly folding wrist, short hand (these traits
make it a much better flier than Archaeopteryx), reptilian traits:
teeth, stomach ribs, unfused hand bones, reptilian-shaped unfused pelvis
(these remaining reptilian traits wouldn't have interfered with flight)
-
"Las Hoyas bird" or "Spanish bird"
(not yet named, early Cretaceous, 131 Ma) -- Another recently found
"little forest flier," still has reptilian pelvis and legs,
with bird-like shoulder
-
Ambiortus dementjevi (early Cretaceous, 125 Ma) -- The third
known "little forest flier," found in 1985
-
Hesperornis, Ichthyornis, and other Cretaceous diving
birds -- This line of birds became specialized for diving, like modern
cormorants, as they lived along saltwater coasts, there are many fossils
known, skeleton further modified for flight (fusion of pelvis bones,
fusion of hand bones, short fused tail), still had true socketed teeth,
a reptilian trait
- the feathered dinosaurs: Sinosauropteryx,
Caudipteryx, Beipiaosaurus, Sinornithosaurus, Microraptor
and many hundreds of specimens of the bird Confuciusornis
  Picture to the left is the famous "Archaeopteryx" --
a
transitional link between reptiles and birds -- there are 10 known specimens
(some are just feathers, some quite complete) -- see the detailed TalkOrigins Archaeopteryx
FAQ -- The exact reptilian ancestor of Archaeopteryx, and
the first development of feathers, are unknown. Early bird evolution
seems to have involved little forest climbers and then little forest
fliers, both of which are guaranteed to leave very bad fossil records (a
little animal with acidic forest soil leaves no remains). Archaeopteryx
itself is really about the best we could ask for: several specimens have
superb feather impressions, it is clearly related to both reptiles and
birds, and it clearly shows that the transition is feasible. The Berlin
specimen is shown at left (discovered in 1877), the Thermopolis
specimen at right (shown under ultraviolet light) is the most recent
[ Source: Science, 2 December 2005 ] with the best preserved feet that are turned
like a dinosaur's (e.g. Velociraptor). Picture to the left is the famous "Archaeopteryx" --
a
transitional link between reptiles and birds -- there are 10 known specimens
(some are just feathers, some quite complete) -- see the detailed TalkOrigins Archaeopteryx
FAQ -- The exact reptilian ancestor of Archaeopteryx, and
the first development of feathers, are unknown. Early bird evolution
seems to have involved little forest climbers and then little forest
fliers, both of which are guaranteed to leave very bad fossil records (a
little animal with acidic forest soil leaves no remains). Archaeopteryx
itself is really about the best we could ask for: several specimens have
superb feather impressions, it is clearly related to both reptiles and
birds, and it clearly shows that the transition is feasible. The Berlin
specimen is shown at left (discovered in 1877), the Thermopolis
specimen at right (shown under ultraviolet light) is the most recent
[ Source: Science, 2 December 2005 ] with the best preserved feet that are turned
like a dinosaur's (e.g. Velociraptor).
From the book Dinosaurs
of the Air: The Evolution and Loss of Flight in Dinosaurs and Birds
by Gregory Paul (John Hopkins Univ Press, 2002) :
"One of the wonderful
coincidences of science is that immediately after Charles Darwin
published On the Origin of Species, his famous explication of
the mechanism behind evolution, dramatic support for his hypothesis
appeared in Bavaria. In 1860, a feather and, in 1861, the skeleton
of a Mesozoic vertebrate obviously intermediate in form between
modern birds and their reptilian ancestors were uncovered in
lithographic slate quarries. This vertebrate was, of course, the
urvogel (original bird) Archaeopteryx. As our knowledge of
fossil birds has expanded in the subsequent fourteen decades, the
question of how birds arose has become ever more fascinating. Most
paleontologists now agree that birds -- always popular with the
public -- happily happen to be the direct descendents of the
best-liked group of extinct creatures, the dinosaurs. Of course,
public opinion has no relevance to scientific debate, but the broad
appeal of a dinosaur-bird link vexes the shrinking minority of
researchers who dispute the link....That birds descended from
predatory dinosaurs has become far and away the majority view
expressed in many additional studies....From China has come the
feathered dinosaurs Sinosauropteryx, Caudipteryx, Beipiaosaurus,
Sinornithosaurus, and Microraptor as well as many
hundreds of specimens of the bird Confuciusornis....the
fossils are coming so fast that it was hard to keep up with the new
data during preparation of this book...." (Gregory Paul, Dinosaurs
of the Air, page 1, 11, 15)
One possible ancestor of Archaeopteryx
is Protoavis (Triassic, 225 Ma) -- A highly controversial fossil
that may or may not be an extremely early bird. Unfortunately, not
enough of the fossil was recovered to determine if it is definitely
related to the birds.
Transitions among mammals
Carnivores
- Creodonts -- early placental
mammals with minor but interestingly carnivore-like changes in the
molars and premolars
-
Cimolestes (late Cretaceous) -- This creodont lost the last molar
and later enlarged the last upper premolar and first lower molar
-
Cimolestes incisus and Cimolestes cerberoides (Cretaceous)
-- These are two species that lost their third molar
-
Cimolestes sp (Paleocene) -- A later, as yet unnamed species that
has very miacid-like teeth
-
Simpsonictis tenuis (mid-Paleocene) -- A very early viverravid
-
Paroodectes, Vulpavus (early Eocene) -- Early miacids, enlarged
carnassials now specialized for shearing
-
Viverravus sicarius (mid-Eocene) -- this viverravid may be the
ancestral aeluroid
Dogs
- Cynodictis (late Eocene) -- First
known arctoid (undifferentiated dog/bear)
-
Hesperocyon (early Oligocene) -- A later arctoid, compared to
miacids like Paroodectes
-
Cynodesmus (Miocene) -- First true dog, the dog lineage continued
through Tomarctus (Pliocene) to the modern dogs, wolves, foxes, Canis
(Pleistocene)
Bears
- Cynodictis (above)
-
Hesperocyon (above)
-
Ursavus elmensis (mid-Oligocene) -- A small, heavy doglike
animal, intermediate between arctoids and bears
-
Protursus simpsoni (Pliocene; also "Indarctos") --
Sheepdog-sized, carnassial teeth have no shearing action, molars are
square, shorter tail, heavy limbs, transitional to the modern genus
Ursus
-
Ursus minimus (Pliocene) -- First little bear, with very bearlike
molars, but still had the first premolars, gave rise to the modern black
bears (U. americanus and U. thibetanus), smoothly evolved to the next
species, U. etruscus
-
U. etruscus (late Pliocene) -- A larger bear, similar to our
brown bear but with more primitive dentition, in Europe gradually
evolved into U. savini
-
U. savini (late Pleistocene, 1 Ma) -- Very similar to the brown
bear
-
U. spelaeus (late Pleistocene) -- The recently extinct giant cave
bear, with a highly domed forehead, clearly derived from the European
population of U. savini in a smooth transition
-
U. arctos (late Pleistocene) -- The brown "grizzly"
bear, clearly derived from the Asian population of U. savini about
800,000 years ago, spread into Europe and the New World
-
U. maritimus (late Pleistocene) -- The polar bear, very similar
to a local population of brown bear, U. arctos beringianus that lived in
Kamchatka about 500,000 years ago
The transitions between each of these
bear species are very well documented. For most of the transitions there
are superb series of transitional specimens leading right across the
species boundaries.
Raccoons (procyonids)
- Phlaocyon (Miocene) -- A climbing
carnivore with non-shearing carnassials and handlike forepaws,
transitional from the arctoids to the procyonids (raccoons), typical
raccoons first appeared in the Pliocene
Weasels (mustelids)
- Plesictis (early Oligocene) --
Transitional between miacids and mustelids (weasels)
-
Potamotherium (late Oligocene) -- Another early mustelid, but has
some puzzling traits
Seals, sea lions and walruses
- Pachycynodon (early Oligocene) --
A bearlike terrestrial carnivore with several sea-lion traits
-
Enaliarctos (late Oligocene, California) -- Still had many
features of bear-like terrestrial carnivores
-
Odobenidae (the walrus family) -- started with Neotherium 14
Ma, then Imagotaria, which is probably ancestral to modern
species
-
Otariidae (the sea lion family), Pithanotaria (mid-
Miocene, 11 Ma) -- small and primitive in many respects
-
Thalassoleon (late Miocene) and finally modern sea lions
(Pleistocene, about 2 Ma)
-
Phocidae (the seal family) -- first known are the primitive and
somewhat weasel-like mid-Miocene seals Leptophoca
- Montherium
-- Modern seals first appear in the Pliocene, about 4 Ma
Civets (viverrids)
- Stenoplesictis (early Oligocene)
-- An early civet-like animal related to the miacids
-
Palaeoprionodon (late Oligocene, 30-24 Ma) -- An aeluroid
(undifferentiated cat/civet/hyena) with a civet-like skull
-
Herpestides (early Miocene, 22 Ma, France) -- Had a distinctly
civet-like skull floor
Cats
- Haplogale (late Oligocene, 30 Ma)
-- A slightly cat-like aeluroid (cat/civet/hyena)
- Proailurus julieni (early Miocene) -- An aeluroid with a
viverrid-ish skull floor that also showed the first cat-like traits
-
Proailurus lemanensis (early Miocene, 24 Ma) -- Considered the
first true cat, had the first really cat-like skull floor
-
Pseudaelurus (early-mid Miocene, 20 Ma) -- A slightly later, more
advanced cat
-
Dinictis (early Oligocene) -- Transitional from early cats such
as Proailurus to modern "feline" cats
-
Hoplophoneus (early Oligocene) -- Transitional from early cats to
"saber-tooth" cats
Rodents
- Anagale, Barunlestes, or
a similar anagalid (mid-late Paleocene) -- A recently discovered
order of primitive rodent/lagomorph ancestors from Asia, rabbit-like
lower cheek teeth, barunlestes in particular (known so far from just
one specimen) has both rodent-like and rabbit-like features, and may
be ancestral to both rodents and lagomorphs, lineage apparently
split into two groups, a eurymyloid/rodent-like group and a
mymotonid/rabbit-like group
- Heomys (mid-late Paleocene,
China) -- An early rodent-like eurymyloid, similar overall to
Barunlestes but with added rodent/lagomorph features
- Tribosphenomys minutus (late
Paleocene, 55 Ma) -- A newer discovery, a small Asian anagalid known
from a single jaw found in some fossilized dung, still had
rabbit-like cheek teeth, but had fully rodent-like ever-growing
first incisors, probably the "ancestral stem" of the
rodents (Discover, Feb 1995, page 22)
- Acritoparamys (was "Paramys")
atavus (late Paleocene) -- first known primitive rodent
- Paramys and ischyromyid group
(late Paleocene) -- Generalized early rodents, mostly squirrel-like
skeleton but without the arboreal adaptations
- Protosciurus (early Oligocene)
-- An early squirrel with very primitive dentition and jaw muscles,
but with the unique ear structure of modern squirrels, fully
arboreal
- Sciurus (Miocene) -- the modern
squirrel genus, among the rodents, squirrels may be considered
"living fossils"
- Paleocastor (Oligocene) --
Early beaver, a burrower, not yet aquatic, modern beavers appear in
the Pleistocene
- Eomyids -- later Eocene rodents
with a few tooth and eyesocket features that show they had branched
off from the squirrel line
- Geomyoids -- primitive rodents
and still have squirrel-like jaws, known to have given rise to the
mouse family
Lagomorphs (rabbits and hares)
- Barunlestes (above) -- The
possible Asian rodent/lagomorph ancestor
- Mimotoma (Paleocene) -- A
rabbit-like animal, similar to Barunlestes, but with a rabbit dental
formula, changes in the facial bones, and only one layer of enamel
on the incisors (unlike the rodents), like rabbits it had two upper
incisors, but the second incisor is still large and functional, in
modern rabbits it is tiny
- Mimolagus (late Eocene) --
Possesses several more lagomorph-like characters, such as a special
enamel layer, possible double upper incisors, and large premolars
- Lushilagus (mid-late Eocene) --
first true lagomorph, teeth very similar to Mimotoma, and modern
rabbit and hare teeth could easily have been derived from these
teeth, after this the first modern rabbits appeared in the Oligocene
Condylarths (first hoofed animals)
- Protungulatum (latest
Cretaceous) -- Transitional between earliest placental mammals and
the condylarths (primitive, small hoofed animals), these early,
simple insectivore-like small mammals had one new development: their
cheek teeth had grinding surfaces instead of simple, pointed cusps,
they were the first mammal herbivores
Within a few million years the
condylarths split into several slightly different lineages with slightly
different teeth, such as oxyclaenids (the most primitive),
triisodontines, and phenacodonts (described in other sections). Those
first differences amplified over time as the lineages drifted further
and further apart, resulting ultimately in such different animals as
whales, anteaters, and horses. Says Carroll (1988): "In the case of
the cetaceans [whales] and the perissodactyls [horses], their origin
among the condylarths has been clearly documented." (page 505) P.D.
Gingerich has analyzed the condylarth lineage and species-to-species
transitions, for example: "Haplomylus speirianus...
gradually became larger over time, ultimately giving rise to a new
species Haplomylus scottianus... Hyopsodus latidens also
became larger and then smaller, ultimately giving rise to a still
smaller species, Hyopsodus simplex." These analyses were
based on hundreds of new specimens (505 for Haplomylus, and 869 for
Hyopsodus) from Clark's Fork Basin in Wyoming.
Perissodactyls (horses, rhinos, tapirs)
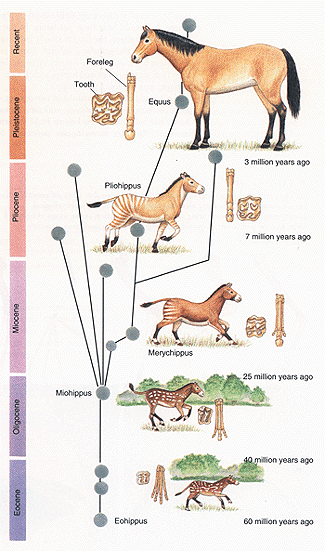 Here we come to the most famous general
lineage of all, the horse sequence. It was the first such lineage to be
discovered, in the late 1800's, and thus became the most famous. It is a
very good sequence that has grown only more detailed and complete over
the years, changing mainly by the addition of large side-branches. Here we come to the most famous general
lineage of all, the horse sequence. It was the first such lineage to be
discovered, in the late 1800's, and thus became the most famous. It is a
very good sequence that has grown only more detailed and complete over
the years, changing mainly by the addition of large side-branches.
- Loxolophus (early Paleocene) --
A primitive condylarth with rather low-crowned molars, probably
ancestral to the phenacodontid condylarths
- Tetraclaenodon (mid-Paleocene)
-- A more advanced Paleocene condylarth from the phenacodontid
family, and almost certainly ancestral to all the perissodactyls (a
different order)
- Radinskya yupingae (late
Paleocene, China) -- A recently discovered perissodactyl-like
condylarth
- Hyracotherium (early Eocene,
about 55 Ma, previously "Eohippus") -- The famous
"dawn horse," a small, doggish perissodactyl, with an
arched back, short neck, omnivore teeth, and short snout, 4 toes in
front and 3 behind
- Hyracotherium vassacciense
(early Eocene) -- The particular species that probably gave rise to
the equids
- Orohippus (mid-Eocene, 50 Ma)
-- Small, 4/3 toed, developing browser tooth crests
- Epihippus (late Eocene, 45 Ma)
-- Small, 4/3 toed, good tooth crests, browser
- Epihippus (Duchesnehippus)
-- A later subgenus with Mesohippus-like teeth
- Mesohippus celer (latest
Eocene, 40 Ma) -- Three-toed on all feet, browser, slightly larger
- Mesohippus westoni (early
Oligocene) -- A slightly later, more advanced species
- Miohippus assiniboiensis
(mid-Oligocene) -- This species split off from early Mesohippus via
cladogenetic evolution, after which Miohippus and Mesohippus
overlapped for the next 4 my
- Kalobatippus (late Oligocene)
-- Three-toed browser w/foot intermediate between Mio and Para
- Parahippus (early Miocene, 23
Ma) -- Three-toed browser/grazer, developing "spring foot"
- Parahippus leonensis
(mid-Miocene, 20 Ma) -- Three-toed browser/grazer with the emphasis
on grazer
- Merychippus gunteri
(mid-Miocene, 18 Ma) -- Three-toed grazer, fully spring-footed with
high-crowned teeth
- Merychippus primus
(mid-Miocene, 17 Ma) -- Slightly more advanced
- Merychippus spp (mid-late
Miocene, 16-15 Ma) -- 3-toed grazers, spring-footed, size of small
pony
The line that eventually produced Equus
developed as follows: M. primus, M. sejunctus, M. isonesus (these last
two still had a mix of primitive, hipparion, and equine features), M.
intermontanus, M. stylodontus, M. carrizoensis. These last two looked
quite horsey, with quite small side toes, and gave rise to a set of
larger three-toed and one-toed horses known as the "true
equines."
- Dinohippus (late Miocene, 12
Ma) -- One-toed grazer, spring-footed, very equine feet, teeth, and
skull, with straighter teeth and smaller fossae, first was D.
spectans, followed by D. interpolatus and D. leidyanus, a slightly
later species was D. mexicanus with even straighter teeth and even
smaller fossae
- Equus (Plesippus), also
called the E. simplicidens group (Pliocene, 4 My) -- Three closely
related species of one-toed spring-footed high-crowned grazers, no
fossae and very straight teeth, pony size, fully "horsey"
body
- Equus (Hippotigris)
(Pleistocene) -- Subgenus of modern 1-toed spring-footed grazing
zebras
- Equus (Equus)
(Pleistocene) -- Subgenus of modern 1-toed spring-footed grazing
horses and donkeys
- Homagalax (early Eocene) --
Very like its sister genus Hyracotherium, but had cross-lophs on
teeth
- Heptodon (late early Eocene) --
A small early tapiroid showing one more tooth cusp change, split
into two lineages
- Helaletes (mid-Eocene) -- which
had a short proboscis
- Prototapir (late Oligocene) --
much like modern tapirs but without such a flexible snout
- Miotapirus (early Miocene) --
an almost-modern tapir with a flexible snout
- Tapirus (Pliocene) -- the
modern tapir
- Hyrachyus (late Eocene) -- a
tapiroid with increased shearing function in its teeth, led to the
late Eocene hyracodontids such as Hyracodon (rhino-tapiroids, or
"running rhinos")
For more details, see the TalkOrigins Horse
Evolution FAQ.
Artiodactyls (pigs, hippos, deer, giraffes, cows)
Carroll (1988) says: "The early evolution
of the artiodactyls is fairly well documented by both the dentition and
considerable skeletal material and provides the basis for fairly detailed
analysis of evolutionary patterns....the origin of nearly all the
recognized families can be traced to the late Middle Eocene or the Upper
Eocene..." (page 507).
- Chriacus (early Paleocene) -- A
primitive oxyclaenid condylarth from the Lower Paleocene
- Diacodexis (early Eocene) -- A
rabbit-sized with longer limbs than the condylarths
- Helohyus or a similar helohyid
(mid-Eocene) -- Primitive artiodactyl, larger than Diacodexis but
with relatively shorter and stouter limbs, with bulbous cusps on the
molars
- Anthracotherium and later
anthracotheriids (late Eocene) -- A group of heavy artiodactyls that
started out dog-size and increased to be hippo-size, later species
became amphibious with hippo-like teeth, led to the modern hippos in
the early Miocene, 18 Ma
- Propalaeochoerus or a similar
cebochoerid/choeropotamid (late Eocene) -- Primitive piglike
artiodactyls derived from the helohyids (above)
- Perchoerus (early Oligocene) -- The
first known peccary
- Paleochoerus (early Oligocene, 38 Ma)
-- first known true pig, apparently ancestral to all modern pigs
- Homacodon and other dichobunids
(mid-Eocene) -- similar to Diacodexis but with some advances
- Poebrodon (late Eocene) -- first
primitive camelid, like other late Eocene artiodactyls, it had
developed crescent-shaped grinding ridges on the cheek teeth, a
small, short-necked, four-toed animal with little hooves on each toe
- Poebrotherium (mid-Oligocene) -- A
taller camelid with fused arm and leg bones, and missing toes 1, 4,
and 5, longer neck, though still much shorter than modern camels
- Mesomeryx (late Eocene) -- A more
advanced dichobunid, probably close to the ancestry of the rest of
the artiodactyls
- Hypertragulus, Indomeryx or a similar
hypertragulid (late Eocene) -- Primitive ruminants with a tendency
toward crescent ridges on teeth, high-crowned teeth, and loss of one
cusp on the upper molars, long-legged runners and bounders, with
many primitive features, but with telltale transitional signs
- Hyemoschus or other tragulids
(Oligocene) -- Slightly more advanced ruminants called "tragulids"
that have the above features plus loss of part of the first toe,
some more bones fused, fibula shaft no longer ossifies
- Archaeomeryx, Leptomeryx (mid-late
Eocene) -- Rabbit-sized ruminants, still had small upper incisors,
the mastoid bone becomes less and less exposed in these "leptomerycids"
- Bachitherium (early Oligocene) -- A
later, more advanced leptomerycid
- Lophiomeryx, Gelocus (late Eocene,
early Oligocene) -- The most advanced ruminants yet, called "gelocids"
with a more compact and efficient ankle, still smaller side toes,
more complex premolars and an almost completely covered mastoid
bone, a slightly different lineage split off from this gelocid
family in the late Eocene or early Oligocene, eventually giving rise
to four families
- Prodremotherium (late Eocene)
-- a
slightly deer-like ruminant
- Eumeryx (Oligocene) -- a more deer-like
ruminant
- Dicrocerus (early Miocene) -- with the
first antlers (similar to living muntjacs)
- Acteocemas (Miocene) and several
successful Miocene and Pliocene groups that survive today as modern
deer -- cervines, white-tails, moose, reindeer
- Climacoceras (very earliest
Miocene) -- first giraffids, then Canthumeryx (also very early Miocene), then
Paleomeryx (early Miocene), then Palaeotragus (early Miocene) a
short-necked giraffid complete with short skin-covered horns
 Samotherium (late Miocene) -- another
short-necked giraffe, and then split into Okapia (one species is
still alive, the okapi, essentially a living Miocene short-necked
giraffe) Samotherium (late Miocene) -- another
short-necked giraffe, and then split into Okapia (one species is
still alive, the okapi, essentially a living Miocene short-necked
giraffe)- Giraffa (Pliocene) -- the modern
long-necked giraffe
- Paracosoryx prodromus (early Miocene,
21 Ma) -- a primitive antilocaprid, probably derived from a North
American branch of the bovid lineage
- Merycodus (Miocene) -- with branched
permanent horns, led to numerous antilocaprids in the Pliocene, only
the pronghorn is still alive
Bovids are known from isolated teeth in
the late Oligocene, then from Eotragus, a primitive ancestral
mid-Miocene bovid. Protragocerus (Miocene) soon followed. The first
sheep (Oioceros) and gazelles (Gazella) are known from the mid-late
Miocene (14 Ma), the first cattle (Leptobos, Parabos) from the early
Pliocene (5 Ma).
text here evolution of giraffes
text

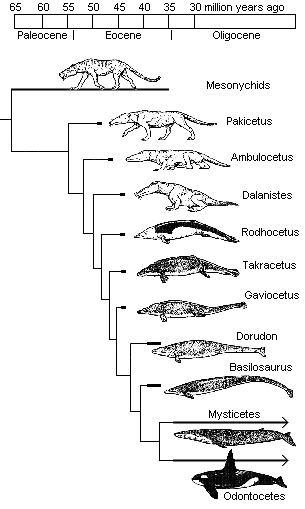 Cetaceans (whales and dolphins) Cetaceans (whales and dolphins)
Just several years ago, there was still a large gap in the fossil
record of the cetaceans. It was thought that they arose from
land-dwelling mesonychids (closely related to the artiodactyls) that gradually lost their hind legs and became
aquatic. Evolutionary theory predicted that they must have gone through
a stage where they were partially aquatic but still had hind legs, but
there were no known intermediate fossils. A flurry of recent discoveries
from India and Pakistan (the shores of the ancient Tethys Sea) has
pretty much filled this gap.
- Eoconodon or similar triisodontine
arctocyonids (early Paleocene)
-
Microclaenodon (mid-Paleocene)
-
Dissacus (mid-Paleocene)
-
Hapalodectes or a very similar
mesonychid (early Eocene, around 55 Ma)
-
Pakicetus (early-mid Eocene, 52 Ma) -- The oldest known whale fossil
- Ambulocetus natans (early-mid
Eocene, 50 Ma) -- A recently discovered early whale, with enough of the
limbs and vertebrae preserved to see how the early whales moved on land
and in the water, this whale had four legs: front legs were stubby, back legs were short but well-developed, with enormous broad feet that
stuck out behind like tail flukes, had no true tail flukes, just a long
simple tail, size of a sea lion, still had a long snout with no
blowhole, probably walked on land like a sea lion, and swam with a
seal/otter method of steering with the front feet and propelling with
the hind feet -- so, just as predicted, these early whales were much like
modern sea lions: they could swim, but they could also still walk on
land (Thewissen
on whale evolution and Science 1994 below)
- Rodhocetus (mid-Eocene, 46 Ma) --
Another very recent (1993) fossil whale discovery, had hind legs a third
smaller than those of A. natans
-
Basilosaurus isis, Protocetes, Indocetus ramani -- small-legged whales mid-late Eocene (45-42 Ma)
-
Prozeuglodon (late Eocene, 40 Ma) -- another recently discovered
whale
-
Eocetus, and similar "archeocete whales" of the late
Eocene
-
Dorudon intermedius -- a late Eocene whale ancestral to modern
whales
Picture below is a reconstruction of the skeleton of Ambulocetus
natans [ Source: Thewissen J.G.M., Hussain S.T., Arif M. "Fossil
evidence for the origin of aquatic locomotion in Archaeocete whales" Science 1994, 263: 210-2 ].

P.D. Gingerich has also done a lot of
work on the evolution of whales. See also this Whale
Evolution PDF by Gingerich (2004). Some of these whale transitional fossils are recent finds (1980s, 1990s), others have been known for 100 years or more. Here is a list
of the "families" of transitionals and when first published or discovered:
- Pakicetidae - include: Pakicetus inachus (1981), Pakicetus attocki (1980), Ichthyolestes pinfoldi (1958), Nalacetus ratimitus (1998)
- Ambulocetidae - include: Ambulocetus natans (1994), Gandakasia potens (1958)
- Remingtoncetidae - include: Remingtoncetus harudiensis (1975), Remingtoncetus sloani (1972), Andrewsiphius kutchensis (1975), Andrewsiphius minor (1975), Dalanistes ahmedi (1995), Attockicetus praecursor (1998)
- Protocetidae - include: Protocetus atavus (1904), Eocetus schweinfurthi (1904), Pappocetus lugardi (1920), Babiacetus mishrai (1998), Rodhocetus kasrani (1994), Takracetus simus (1995), Georgiacetus vogtlensis (1998)
- Basilosaurids - include: Basilosaurus cetoides (1834), Basilosaurus isis (1906), Basilosaurus drazindai (1997), Basiloterus hussaini (1997)
- Dorudontines - include: Dorudon serratus (1845), Dorudon atrox (1906)
-
others - Pontogeneus brachyspondylus (1849), Zygorhiza kochii (1847), Saghacetus osiris (1894), Ancalacetus simonsi (1996)
From Thewissen's book The Emergence of Whales: Evolutionary Patterns in the Origin of Cetacea (Springer / Plenum Press,
1998), a quote from the beginning of chapter 14: "Isotopic Approaches to Understanding the Terrestrial-to-Marine Transition of the Earliest Cetaceans"
:
"The fossil record is replete with examples of evolutionary transitions between marine and freshwater environments, in both directions. Perhaps the most striking and best documented example of such a transition is the evolution of cetaceans (whales, dolphins, and porpoises) from the extinct group of terrestrial mammals called mesonychians." (J.G.M. Thewissen, editor,
The Emergence of Whales, chapter 14)
There are now about 30-40 whale intermediates, and according to the
most modern research the common ancestor is seen to be derived from the artiodactyls (Even-Toed ungulates) rather than
mesonychids. [ Source: "Whales originated from aquatic artiodactyls in the Eocene epoch of India"
by J.G.M. Thewissen, L.N. Cooper, M.T. Clementz, S. Bajpai & B.N. Tiwari
in
Nature 450, 1190-1194 (20 December 2007) ]. Wikipedia has a good overview of cetacean
evolution:
"The traditional theory of cetacean evolution was that whales were related to the
mesonychids, an extinct order of carnivorous ungulates (hoofed animals), which looked rather like wolves with hooves and were a sister group of artiodactyls. These animals possessed unusual triangular teeth that are similar to those of whales. For this reason, scientists had long believed that whales evolved from a form of
mesonychid." (from the
Wikipedia
article on "Evolution of Cetaceans")
In the Oligocene (between 34 and 24 Ma), whales split into two
lineages:
-
Toothed: Agorophius (late Oligocene), Prosqualodon (late
Oligocene), Kentriodon (mid-Miocene)
-
Baleen (Toothless): Aetiocetus (late Oligocene), Mesocetus
(mid-Miocene) lost its teeth, modern baleen whales first appeared in the late
Miocene
Video of Nature article Dec 2007
with JGM Thewissen
Video
of Whale evolution with Phil Gingerich from PBS Evolution series
(2001)
Sirenians (dugongs and manatees)
- Early Eocene -- fragmentary sirenian
fossils known from Hungary
- Prorastomus (mid-Eocene) -- A
very primitive sirenian with an extremely primitive dental formula
(including the ancient fifth premolar that all other mammals lost in
the Cretaceous), this could mean sirenians split off from all other
mammals very early on, skull is somewhat condylarth-like, had
distinctive sirenian ribs
- Protosiren (late Eocene) -- A
sirenian with an essentially modern skeleton, though it still had
the very primitive dental formula, probably split into the two
surviving lineages
- Dugongs: Eotheroides (late
Eocene), with a slightly curved snout and small tusks, still with
the primitive dental formula
- Halitherium (Oligocene) -- a
dugong-ish sirenian with a more curved snout and longer tusks, and
then to living dugongs, very curved snout and big tusks
- Manatees: Sirenotherium (early
Miocene)
- Potamosiren (late Miocene) -- a
manatee-like sirenian with loss of some cheek teeth
- Ribodon (early Pliocene) -- a
manatee with continuous tooth replacement, and then the living
manatees
 Picture to the left is an intermediate Sirenian fossil: a seacow
with legs. Reconstructed skeleton of Pezosiren portelli. Length
is approximately 7 feet. Gray coloring represents extant fossils, white
elements are partly conjectural. [ Source: Domning, D. P. (2001) "The
earliest known fully quadrupedal sirenian" Nature 413:
625-627 ] Picture to the left is an intermediate Sirenian fossil: a seacow
with legs. Reconstructed skeleton of Pezosiren portelli. Length
is approximately 7 feet. Gray coloring represents extant fossils, white
elements are partly conjectural. [ Source: Domning, D. P. (2001) "The
earliest known fully quadrupedal sirenian" Nature 413:
625-627 ]
|
Elephants
Picture to the right is a graphic representation of the elephant (proboscidean)
evolutionary lineage. The fossil record of their ancestors goes back 50
million years. [ Source: Miller, Finding Darwin's God, page
96 ]
- Minchenella or a similar condylarth (late
Paleocene) -- Known only from lower jaws
-
Phenacolophus (late Paleocene or early
Eocene)
-
Pilgrimella (early Eocene)
-
Moeritherium, Numidotherium, Barytherium
(early-mid Eocene) -- A group of three similar very early elephants,
pig-sized with stout legs,
broad spreading feet and flat hooves, no trunk
-
Paleomastodon, Phiomia (early Oligocene)
-- The first "mastodonts," medium-sized animals with a trunk
-
Gomphotherium (early Miocene) --
Basically a large edition of Phiomia
- Stegotetrabelodon (late Miocene) -- One
of the first of the "true" elephants, but still had two long
rows of cross-crests, functional premolars, and lower tusks
-
Primelephas (latest Miocene) -- Short
lower jaw makes it look like an elephant now
-
Primelephas gomphotheroides
(mid-Pliocene) -- A later species that split into three lineages, Loxodonta,
Elephas, and Mammuthus
In general, after the earliest forms of the three modern
genera appeared, they show very smooth, continuous evolution
with almost half of the speciation events preserved in fossils.
For instance, Carroll (1988) says: "Within the genus Elephas, species demonstrate continuous change over a period of
4.5 million years....the elephants provide excellent evidence of
significant morphological change within species, through species
within genera, and through genera within a family." (page
575)
|
|
Primates (and Hominids)
A little more controversial, here is the
outline of the lineage that led to humans (homo sapiens sapiens).
For more detailed information see the TalkOrigins Fossil
Hominids FAQ.
- Palaechthon, Purgatorius
(middle Paleocene) -- Very primitive plesiadapids, to modern eyes
they look nothing like primates, being simply pointy-faced, small
early mammals with mostly primitive teeth, and claws instead of
nails, but they show the first signs of primate-like teeth, lost an
incisor and a premolar, and had relatively blunt-cusped, squarish
molars
- Cantius (early Eocene) -- One
of the first true primates (or "primates of modern
aspect"), more advanced than the plesiadapids (more teeth lost,
bar behind the eye, grasping hand and foot) and beginning to show
some lemur-like arboreal adaptations
- Pelycodus and related species
(early Eocene) -- Primitive lemur-like primates
- Amphipithecus, Pondaungia
(late Eocene, Burma) -- Very early Old World primates known only
from fragments, larger brain, shorter nose, more forward-facing eyes
(halfway between plesiadapid eyes and modern ape eyes)
- Parapithecus (early Oligocene)
-- The OW monkeys split from the apes around now, this was
probably at the start of the OW monkey line, from here the OW
monkeys go through Oreopithecus (early Miocene, Kenya) to
modern monkey groups of the Miocene and Pliocene
- Propliopithecus, Aegyptopithecus
(early Oligocene, Egypt) -- From the same time as Parapithecus, but
probably at the beginning of the ape lineage, first ape characters
(deep jaw, 2 premolars, 5-cusped teeth)
- Aegyptopithecus (early-mid
Oligocene, Egypt) -- Slightly later anthropoid (ape/hominid) with
more ape features, was a fruit-eating runner/climber, larger, with a
rounder brain and shorter face
- Proconsul africanus (early
Miocene, Kenya) -- A sexually dimorphic, fruit-eating, arboreal
quadruped probably ancestral to all the later apes and humans,
mosaic of ape-like and primitive features, ape-like elbow, shoulder
and feet, monkey-like wrist, gibbon-like lumbar vertebrae
- Limnopithecus (early Miocene,
Africa) -- A later ape probably ancestral to gibbons
- Dryopithecus (mid-Miocene) -- A
later ape probably ancestral to the great apes and humans, at this
point Africa and Asia connected via Arabia, and the non-gibbon apes
divided into two lines
- Sivapithecus (e.g. "Gigantopithecus"
and "Ramapithecus" mid-Miocene) -- moved to Asia and gave
rise to the orangutan
- Kenyapithecus (mid-Miocene,
about 16 Ma) -- stayed in Africa and gave rise to the African great
apes and humans
- Australopithecus ramidus
(mid-Pliocene, 4.4 Ma) -- A recently discovered very early hominid
(or early chimp), from just after the split with the apes, possibly
bipedal (only the skull was found), teeth both apelike and humanlike
- Australopithecus afarensis
(late Pliocene, 3.9 Ma) -- some excellent fossils ("Lucy")
make clear that this was fully bipedal and definitely a hominid, an
extremely ape-like hominid, only four feet tall, still had an
ape-sized brain of just 375-500 cc (finally answering the question
of which came first, large brain or bipedality) and ape-like teeth
- Australopithecus africanus
(later Pliocene, 3.0 Ma) -- the more slender lineage, up to five
feet tall, with slightly larger brain (430-550 cc) and smaller
incisors, teeth gradually became more and more like Homo teeth,
these hominids are almost perfect ape-human intermediates, now pretty clear that the slender australopithecines led to the
first Homo species
- Homo habilis (latest
Pliocene/earliest Pleistocene, 2.5 Ma) -- Straddles the boundary
between australopithecines and humans, sometimes lumped with the
australopithecines, about five feet tall, face still primitive but
projects less, molars smaller, brain 500-800 cc, overlapping
australopithecines at the low end and early Homo erectus at the
high end, perhaps capable of rudimentary speech, first clumsy stone
tools
- Homo erectus (e.g. "Java
Man," "Peking Man," "Heidelberg Man,"
Pleist, 1.8 Ma) -- Looking much more human now with a brain of
775-1225 cc, but still has thick brow ridges and no chin, spread out
of Africa and across Europe and Asia, good tools, first fire
- Archaic Homo sapiens
(Pleist, 500,000 years ago) -- These first primitive humans were
perfectly intermediate between H. erectus and modern humans,
with a brain of 1200 cc and less robust skeleton and teeth, over the
next 300,000 years brain gradually increased, molars got still
smaller, skeleton less muscular, clearly arose from H. erectus but
there are continuing arguments about where this happened
- Homo sapiens sapiens (e.g.
"Cro-magnons" late Pleist, 40,000 years ago) -- All modern
humans, average brain size 1350 cc, in Europe gradually
supplanted the Neandertals
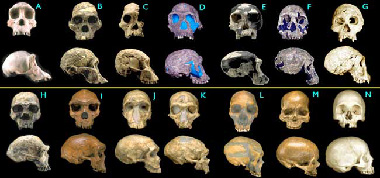 Picture
at right includes some hominid fossils: (A) Pan troglodytes,
modern chimpanzee; (B)
Australopithecus africanus, 2.6 My; (C) Australopithecus
africanus, 2.5 My; (D) Homo habilis, 1.9 My;
(E) Homo habilis, 1.8 My; (F) Homo rudolfensis, 1.8 My; (G) primitive
Homo erectus, Dmanisi cranium, 1.75 My;
(H) Homo ergaster (late H. erectus), 1.75 My; (I) Homo
heidelbergensis, "Rhodesia man," 300,000 - 125,000 y;
(J) Homo sapiens neanderthalensis, 70,000 y; (K) Homo sapiens
neanderthalensis, 60,000 y;
(L) Homo sapiens neanderthalensis, 45,000 y; (M) Homo sapiens
sapiens, Cro-Magnon, 30,000 y;
(N) modern Homo sapiens sapiens. Picture
at right includes some hominid fossils: (A) Pan troglodytes,
modern chimpanzee; (B)
Australopithecus africanus, 2.6 My; (C) Australopithecus
africanus, 2.5 My; (D) Homo habilis, 1.9 My;
(E) Homo habilis, 1.8 My; (F) Homo rudolfensis, 1.8 My; (G) primitive
Homo erectus, Dmanisi cranium, 1.75 My;
(H) Homo ergaster (late H. erectus), 1.75 My; (I) Homo
heidelbergensis, "Rhodesia man," 300,000 - 125,000 y;
(J) Homo sapiens neanderthalensis, 70,000 y; (K) Homo sapiens
neanderthalensis, 60,000 y;
(L) Homo sapiens neanderthalensis, 45,000 y; (M) Homo sapiens
sapiens, Cro-Magnon, 30,000 y;
(N) modern Homo sapiens sapiens.
One famous offshoot group, the
Neandertals (other spelling is Neanderthals), developed in Europe about
500,000 years ago. They are considered
to be the same species as us, but a different subspecies, Homo
sapiens neanderthalensis. They were more muscular, with a slightly
larger brain of 1450 cc, a distinctive brow ridge, and differently
shaped throat (possibly limiting their language). They are known to have
buried their dead.
"The current fossil, molecular,
and archeological evidence strongly supports the specific distinction
of the Neanderthals from early and modern H. sapiens. The
Neanderthal lineage first appeared some 500,000 years ago, according
to the molecular clock, and the fossil record suggests that the 'Steinheim
group' reflects a basal Neanderthal anatomical condition. This group
exploited the colder conditions of an Ice Age Europe, their survival
in this region enabled by the increasing development of their 'cold
exaptations.' This initial split between these two groups was not
based on a greater ability to withstand Ice Age conditions (though
ultimately this must have pushed them farther apart over time), but
occurred for some other reason, perhaps cultural....The molecular
evidence shows that Neanderthal mtDNA [mitochondrial DNA] is
significantly different not only from modern humans, but also from homo
sapiens dating back to 40,000 years ago....By 30,000 years ago,
increased competition, increased mortality rates, and a declining
birthrate sent the Neanderthals in a downward spiral to extinction.
There is no need to appeal to an argument of 'prehistoric genocide' to
explain their final disappearance from the earth some 27,000 years
ago." (from Bones, Stones, and Molecules by Cameron /
Groves [Elsevier Academic Press, 2004], page 283-284)
Adapted from the detailed TalkOrigins "Transitional
Vertebrate Fossils FAQ" (1994, 1997) by Kathleen Hunt, and
updated from the book Evolution: What the Fossils Say and Why It
Matters by paleontologist / geologist Donald R. Prothero
(Columbia Univ Press, 2007).
Reply to a Catholic Creationist (updated
December 2008)
See also Catholic
Views on Evolution and Creation
 |
Picture: classic Christian Fish
symbol for Jesus Christ, Son of God, Savior (in Greek), and
Darwin Fish mockery: Darwin, Son of Satan, Send us to hell? |
|
Now I would like to answer a few objections from a Catholic creationist
I received in Email. The creationist states:
<< I am no scientist, nor do I wish to argue
"science" with you. I am a Christian and I believe
Revelation over science. Truth cannot contradict truth. Therefore, if
science contradicts the Holy Writ, I do not believe science. My only
point in referring you to Dr. Humphries was that there are alternate
theories. When most of the bishops were Arians, Athanasius was still
right. If you wish to deform the Text of which God Himself is the
Author in order to accomodate man-made theories, that is your
perogative. Only, such a conviction on your part demands a greater
conviction than my own. >>
This shows an appalling lack of appreciation for modern science. It's
not a matter of "deforming" the Bible, it's a matter how we
understand the first chapters of Genesis. There
are many plausible views held by Christian scholars. The modern
Popes (i.e. Pius XII, JPII, Benedict XVI) have argued a theistic evolutionary
framework is compatible with the Catholic faith (along with other
scientists who are Catholic -- physicist Fr. Stanley
Jaki, astronomer
Fr. George Coyne, other members of the Pontifical
Academy of Sciences, etc). I find most
creationists who reject evolution wholesale really know nothing of the science
that's behind the theory. It seems your typical creationist has read a few
creationist books or culled objections from a few creationist web sites,
but rarely if ever do they read a modern book of science (biology, paleontology,
geology, etc) by an evolutionist (which are basically all professional
scientists). That's as fair
as reading anti-Catholic fundamentalist literature to arrive at an accurate
understanding of the Catholic faith. What one needs to do is examine the
real science and what the evolutionists have to say for themselves. What
is the evidence they present? I have done that (summarized above), and
find it very convincing. Truth cannot contradict truth, and if the
evidence points in the direction of an old earth and evolution, we should be committed to that truth wherever its
found. Science is not about "proof" or absolutes, but a
scientific theory advances and becomes more carefully understood as new evidence and research is found.
Pope John Paul II in his 1996 address to the Pontifical Academy of
Sciences declared evolution to be "more than a hypothesis"
and that a significant argument in favor of the theory is that it has
been progressively accepted by researchers following
independently conducted scientific work in various fields. As the Catechism states:
"...the origins of the world and of man has been the object
of many scientific studies which have splendidly enriched our
knowledge of the age and dimensions of the cosmos, the development of
life-forms and the appearance of man. These discoveries invite us to
even greater admiration for the greatness of the Creator..."
(paragraph 283)
Neither the Catechism nor the Pope suggest we should abandon modern
science and turn to fundamentalist "creation scientists" who
have no credibility in the scientific world. Humphreys is a Protestant
young-earther who is not taken seriously by anyone in conventional
science. Why a Catholic would refer to him I do not know.
<< My unsolicited advice to you: Do not throw
caution to the wind, and show some moderation. These are only theories
-- theories which contradict divine revelation. Listen, then, to the
words of Saint Augustine...Augustine does not say that we should
reconcile our Scriptures with their theories, but that we must
reconcile their theories to our Scriptures. If their theories are
unreconcilable with our Scriptures, then believe the theories to be
false, not the Scriptures! Stop deforming the Sacred Writ to conform
to "scientific" theories, and start making scientific
theories conform to the Holy Scriptures. That is what Dr. Humphries
the Fundamentalist has done. It is time we Catholics did the same.
>>
This shows a misunderstanding of what a theory is -- macroevolution
is considered both a fact and a theory, just as gravity. Macroevolution happened
(fact) and we know that from the lines of evidence presented above, but how it
precisely happened (theory) and does happen are debated among
scientists. A scientific theory is defined as above a mere hypothesis (i.e. evolution
is "more than a hypothesis") and includes, incorporates,
unites, and explains observable
facts and laws.
Since Genesis can be plausibly interpreted a number of ways, theistic
evolution does not contradict divine revelation.
 << If the First Adam is a myth, so is the
Second Adam. Think about that long and hard....If a scientist were to commit sacrilege and examine a
consecrated Host, what do you think he would tell you about it? Yet
you know he is absolutely, irrefutably wrong. Why, then, is he so
infallibly right when it comes to the creation of the Universe and of
man? Has not God told you just as plainly -- just as literally! -- of
how He miraculously created the Universe and man, as He has told you
of the miracle of Transubstantiation? Science refutes them both. Why,
then, do you believe Him on the one and doubt Him on the other?
Because of science? What kind of faith is that? And where is the
consistency? >> << If the First Adam is a myth, so is the
Second Adam. Think about that long and hard....If a scientist were to commit sacrilege and examine a
consecrated Host, what do you think he would tell you about it? Yet
you know he is absolutely, irrefutably wrong. Why, then, is he so
infallibly right when it comes to the creation of the Universe and of
man? Has not God told you just as plainly -- just as literally! -- of
how He miraculously created the Universe and man, as He has told you
of the miracle of Transubstantiation? Science refutes them both. Why,
then, do you believe Him on the one and doubt Him on the other?
Because of science? What kind of faith is that? And where is the
consistency? >>
A couple of objections are made here. First of all, Adam would not be
a "myth" since evolutionary theory would seem to trace our
ancestors back to an original man and woman (let's call them
"Adam" and "Eve" as Genesis does* [see
note below]). Whether Adam
received his body through an evolutionary development is a matter for
science to determine (see the large TalkOrigins
Fossil Hominids site by Jim Foley). God would create the soul in
that first man and woman. So I don't deny there was an original
"Adam" or first man who had his soul created by God nor a Fall
of those first human parents as the Catholic faith defines and explains
(see
my long article on Original Sin). I simply do not interpret all of
Genesis literally (e.g. the talking snake or serpent in Genesis 3).
Though I'll admit there are seemingly good theological objections to the
evolution of man that need to be answered, there are no good scientific
ones.
The above also confuses science with faith. We don't learn from
science that the Eucharist is the body and blood of Christ (i.e.
transubstantiation), we take that on faith based on revelation
(Scripture and Tradition) and the authority of the
Catholic Church who interprets that revelation.
Science would have nothing to say about that, nor about miracles in
general. So here we're confusing faith and divinely revealed doctrine with science. Also,
science is not about "absolutes" or "proof" but
improves as we move forward in research, discoveries, and technology.
Scientists themselves are certainly not infallible, they have made many
mistakes. But it is once again science and other scientists using the
"scientific method" that corrects them.
The theory of evolution is the best we have from modern science today
that explains the development of plants, animals, and man. If one
rejects evolution wholesale, what comprehensive scientific theory of
"creationism" would one substitute in its place that explains the evidence and data we find from the natural history of our world?
see also Part 1: The Scientific Evidence for an Old Earth
*note: So I don't misrepresent the science, here is
what the latest genetics evidence tells us about the real
"Adam" and "Eve":
"....the fact that a single ancestor gave rise
to all of the diversity present today does not mean that this was the
only person alive at the time -- only that descendent lineages of the
other people alive at the same time died out.....we are all the recent
descendents of a single woman who lived in Africa less than 150,000
years ago. This result begs the question of where Eve actually lived
-- where in Africa was the Garden of Eden? In one sense this is a red
herring, since we know that there were many women alive all over
Africa at this time.....the root of the male family tree was placed in
Africa -- exactly the same answer that mtDNA had given us for women.
The shocker came when a date was estimated for the age of the oldest
common ancestor. This man, from whom all men alive today ultimately
derive their Y-chromosomes, lived 59,000 years ago. More than 80,000
years after that estimated for Eve! Did Adam and Eve never meet? No
they didn't, but the reason is fairly complicated....." (The
Journey of Man: A Genetic Odyssey by Spencer Wells [Random House
Paperback, 2003], pages 32, 40, 54)
Author Spencer Wells also comments in an interview
for Princeton University Press:
How does the genetic Adam relate to the Adam of the Bible?
"It's interesting that both genetics and the Bible show that there is a common origin of humanity. According to genetic data we come from a single male ancestor. In the Bible too it is mentioned that there is a single male Adam and single female, Eve. I don't equate our results one-to-one with the biblical story, of course, because if you count back through the generations described in the Bible, Adam should have existed in 4004 BC, and our Adam existed 60,000 years ago. Also, our Adam and Eve weren't the only people alive at the time, just the lucky ones who left descendants down to the present day. But it is nice to know that we arrive at the same general conclusion: we're all related."
(from an interview with geneticist Spencer Wells)
Possible Scenario (updated December 2008)
The following is a possible scenario how Adam/Eve could be reconciled
with a population of early hominids/humans.
<< I just quoted decrees from the Council of Trent which are binding on all Catholics, which clearly state that Adam was "the first man", and "the whole human race" is descended from him. >>
So, how can we combine the evidence present in the world that God created with the given interpretation of God's word?
I start from the assumption that a "human" has a human soul, whereas a non-human does not. I also assume that a human soul is immaterial and its presence has no visible material effect, such as a change in DNA.
Here is one possibility. Start with a population of unsouled upright apes, call then
"huma" because they are not quite human yet. God puts human souls into two of them, Adam and
Eve (or puts a soul into one male, Adam, and clones a female, Eve, from
him e.g. Genesis 2:21-23 "Eve from Adam"). Adding a soul does not change the original
huma DNA at all. We now have a pair of humans, Adam and Eve, in a population of
huma. Adam and Eve only mate with each other and have human children with souls. In order to avoid incest the children need to find mates outside their immediate family so they mate with some of the
huma. This is possible because their DNA is compatible with huma DNA; the mating is open to the possibility of creating life.
God gives a soul to all hybrid human/huma offspring so all the children with at least one human parent are also human, i.e. they have a soul. Because only the descendants of the initial pair mate with
huma, all the children from such matings are descended from both Adam and Eve since they will have both as grandparents, great-grandparents etc.
Over time the number of humans increases and the number of huma declines until the
huma are extinct.
In scientific terms we have a large interbreeding population, as shown by the current level of genetic diversity in humans. Theologically all humans are descended from that first ensouled pair, as required by the Council or Trent.
You may or may not accept this particular scenario, but it shows that there is a way to reconcile revelation and science. It also avoids the problem of incest among Adam and Eve's children.
Rossum (a buddhist from Catholic Answers forums)
For more see also Adam,
Eve, and the Hominid Fossil Record
also Hominization:
Origin of Mankind, and a Story of the Fall
BIBLIOGRAPHY (updated July
2010)
ORIGIN OF LIFE AND EVOLUTION OF THE "BIG PICTURE"
- Why Evolution is True by Jerry
A. Coyne (Penguin Books, 2009)
- The Evolution Revolution: design without intelligence by Ken McNamara & John
Long (Melbourne Univ Press, 2007)
- Evolution edited by In-Young Chang and Jennifer Curry
(H.W. Wilson, 2006)
- Genesis: The Scientific Quest for
Life's Origin by Robert M. Hazen (Joseph Henry Press, 2005)
- Evolution: The Remarkable History
of a Scientific Theory by Edward J. Larson (Modern Library,
2004)
- Life's Solution: Inevitable Humans
in a Lonely Universe by Simon Conway Morris (Cambridge Univ
Press, 2003)
- Aquagenesis: the origin and evolution of life in the sea by Richard Ellis
(Viking, 2001)
- Evolution: The Triumph of an Idea by Carl Zimmer
(HarperCollins, 2001)
- A Walk Through Time: from stardust to us : the evolution of life on earth by
Liebes, Sahtouris, Swimme (Wiley, 1998)
- The Origin of Species by
Charles Darwin (Signet Classic, 2003, orig 1859)
EVOLUTION, PHILOSOPHY, AND CHRISTIANITY
(CATHOLICISM)
- Creation or Evolution? Do We Have
to Choose? by Denis Alexander (Monarch Books, 2008)
- Creation and Evolution: A
Conference with Pope Benedict XVI (Ignatius Press, 2008)
- Chance or Purpose? Creation,
Evolution and a Rational Faith by Cardinal Christoph Schonborn
(Ignatius Press, 2007)
- Intelligent Design: William Dembski and Michael Ruse in Dialogue (Fortress Press, 2007)
- Debating Design: From Darwin to DNA
edited by William Dembski and Michael Ruse (Cambridge Univ Press,
2004)
- Perspectives on an Evolving
Creation edited by Keith Miller (Eerdmans, 2003)
- Responses to 101 Questions on God
and Evolution by John F. Haught (Paulist Press, 2001)
- Intelligent Design: The Bridge
Between Science and Theology by William Dembski (Intervarsity
Press, 1999)
- Finding Darwin's God: A Scientist's
Search for Common Ground Between God and Evolution by Kenneth R.
Miller (HarperCollins, 1999)
- Only a Theory: Evolution and the
Battle for America's Soul by Kenneth R.
Miller (Viking Adult, 2008)
- Darwin's Forgotten Defenders: The
Encounter Between Evangelical Theology and Evolutionary Thought
by David N. Livingstone (Eerdmans, 1987)
- The Post-Darwinian Controversies
by James R. Moore (Cambridge Univ Press, 1981, 1979)
INVERTEBRATE EVOLUTION
- An Introduction to the
Invertebrates by Janet Moore (Cambridge Univ Press, 2006)
- On the Origin of Phyla by James W.
Valentine (Univ of Chicago Press, 2004)
- Invertebrate Palaeontology and
Evolution by E.N.K. Clarkson (Blackwell Science, 1998)
EVOLUTION OF ARTHROPODS (INCLUDING
INSECTS)
- Evolution of the Insects by David
Grimaldi and Michael S. Engel (Cambridge Univ Press, 2005)
- Crustacea and Arthropod
Relationships edited by Stefan Koenemann and Ronald A. Jenner (Taylor & Francis,
2005)
- Arthropod Fossils and Phylogeny edited by Gregory D.
Edgecombe (Columbia Univ Press, 1998)
- The Evolution of Insects by Philip S. Callahan
(Holiday House, 1972)
VERTEBRATE EVOLUTION
- Vertebrate Paleontology by
Michael J. Benton (Wiley-Blackwell, 2004, 3rd edition)
- Patterns and Processes of
Vertebrate Evolution by Robert L. Carroll (Cambridge Univ Press,
1997)
- Vertebrate Paleontology and
Evolution by Robert L. Carroll (Freeman, 1988)
EVOLUTION OF TETRAPODS (FOUR-LEGGED
"AMPHIBIANS")
- Your Inner Fish: A Journey into the
3.5-Billion-Year History of the Human Body by Neil Shubin
(Pantheon, 2008)
- Gaining Ground: the origin and evolution of tetrapods by Jennifer A.
Clack (Indiana Univ Press, 2002)
- At the Water's Edge: Fish with Fingers, Whales with Legs, and How Life Came Ashore but Then Went Back to Sea by Carl Zimmer
(Free Press, 1999)
EVOLUTION OF MAMMALS (INCLUDING WHALES)
- The Rise of Placental Mammals: origins and relationships of the Major Extant Clades edited by Kenneth D. Rose and J. David
Archibald (Johns Hopkins Univ Press, 2005)
- The Origin and Evolution of Mammals by
T.S. Kemp (Oxford Univ Press, 2005)
- Morphological Change in Quaternary Mammals of North America
edited by Robert A. Martin and Anthony D. Barnosky (Cambridge Univ Press,
2005)
- Beasts of Eden: walking whales, dawn horses, and other enigmas of mammal evolution by David Rains
Wallace (Univ of CA Press, 2004)
- Mammals from the Age of Dinosaurs: origins, evolution, and structure by Zofia
Kielan-Jaworowska, Richard L. Cifelli, and Zhe-Xi
Luo (Columbia Univ Press, 2004)
- Late Cretaceous and Cenozoic Mammals of North America
-- Biostratigraphy and Geochronology edited by Michael O. Woodburne
(Univ of CA Press, 2004)
- Horns, Tusks, and Flippers: the evolution of hoofed mammals by Donald R. Prothero and Robert M.
Schoch (Johns Hopkins Univ Press, 2002)
- The Emergence of Whales: evolutionary patterns in the origin of Cetacea edited by
J.G.M. Thewissen (Plenum Press, 1998)
- Fossil Horses: Systematics, Paleobiology, and Evolution of the Family Equidae by Bruce J. MacFadden (Cambridge Univ Press, 1994)
- The Evolution of Perissodactyls by Donald R. Prothero and Robert
M. Schoch, ed. (Oxford Univ Press, 1989)
EVOLUTION OF BIRDS
- The Inner Bird: anatomy and evolution by Gary W.
Kaiser (Vancouver: UBC Press, 2007)
- Dinosaurs of the Air : the evolution and loss of flight in dinosaurs and birds by Gregory S.
Paul (Johns Hopkins Univ Press, 2002)
- Taking Wing : Archaeopteryx and the evolution of bird flight by Pat
Shipman (Simon & Schuster, 1998)
- The Rise of Birds : 225 million years of evolution by Sankar
Chatterjee (Johns Hopkins Univ Press, 1997)
- The Origin and Evolution of Birds by Alan
Feduccia (Yale Univ Press, 1996)
HUMAN EVOLUTION
- The First Human: The Race to
Discover our Earliest Ancestors by Ann Gibbons (Doubleday, 2006)
- Bones, Stones, and Molecules: "Out of Africa" and Human Origins by David W. Cameron and Colin P. Groves
(Elsevier, 2004)
- The Journey of Man: A Genetic
Odyssey by Spencer Wells (Random House paperback, 2003)
-
The Human Fossil Record (volume 1, forthcoming in 4 volumes) by Schwartz / Tattersall (John Wiley and Sons, 2002)
-
Extinct Humans by Ian Tattersall and Jeffrey H. Schwartz (Westview Press / Perseus Books, 2000)
-
The Cambridge Encyclopedia of Human Evolution edited by Steve Jones, Robert Martin, David Pilbeam (Cambridge Univ Press, 1992)
-
The Search for Eve by Michael H. Brown (Harper and Row, 1990)
-
Guide to Fossil Man by Michael H. Day (Univ of Chicago Press, 1986, 4th edition)
-
Lucy: The Beginnings of Humankind by Donald C. Johanson and Maitland A. Edey (Simon and Schuster, 1981)
see also Part 1: The Scientific Evidence for an Old Earth
International Theological Commission on Creation and Evolution
The Talk Origins Age of the Earth FAQs
http://www.talkorigins.org/origins/faqs-youngearth.html
The Talk Origins Evolution FAQs
http://www.talkorigins.org/origins/faqs-evolution.html
The Full Transcript and Audio of the Firing Line 1997 Creation-Evolution Debate
P
|
|
 Picture
at right includes some hominid fossils: (A) Pan troglodytes,
modern chimpanzee; (B)
Australopithecus africanus, 2.6 My; (C) Australopithecus
africanus, 2.5 My; (D) Homo habilis, 1.9 My;
(E) Homo habilis, 1.8 My; (F) Homo rudolfensis, 1.8 My; (G) primitive
Homo erectus, Dmanisi cranium, 1.75 My;
(H) Homo ergaster (late H. erectus), 1.75 My; (I) Homo
heidelbergensis, "Rhodesia man," 300,000 - 125,000 y;
(J) Homo sapiens neanderthalensis, 70,000 y; (K) Homo sapiens
neanderthalensis, 60,000 y;
(L) Homo sapiens neanderthalensis, 45,000 y; (M) Homo sapiens
sapiens, Cro-Magnon, 30,000 y;
(N) modern Homo sapiens sapiens.
Picture
at right includes some hominid fossils: (A) Pan troglodytes,
modern chimpanzee; (B)
Australopithecus africanus, 2.6 My; (C) Australopithecus
africanus, 2.5 My; (D) Homo habilis, 1.9 My;
(E) Homo habilis, 1.8 My; (F) Homo rudolfensis, 1.8 My; (G) primitive
Homo erectus, Dmanisi cranium, 1.75 My;
(H) Homo ergaster (late H. erectus), 1.75 My; (I) Homo
heidelbergensis, "Rhodesia man," 300,000 - 125,000 y;
(J) Homo sapiens neanderthalensis, 70,000 y; (K) Homo sapiens
neanderthalensis, 60,000 y;
(L) Homo sapiens neanderthalensis, 45,000 y; (M) Homo sapiens
sapiens, Cro-Magnon, 30,000 y;
(N) modern Homo sapiens sapiens.
 The Evidence for Evolution
The Evidence for Evolution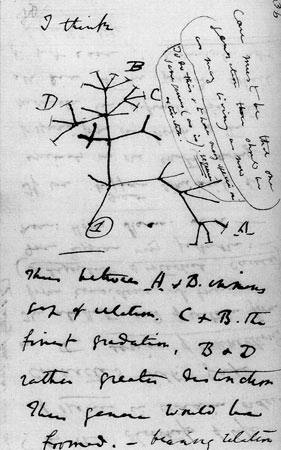



 MESOZOIC
MESOZOIC  The following data is adapted from the detailed TalkOrigins "Transitional
Vertebrate Fossils FAQ" (1994-1997) by Kathleen Hunt, and
updated from the book Evolution: What the Fossils Say and Why It
Matters by paleontologist / geologist Donald R. Prothero
(Columbia Univ Press, 2007).
The following data is adapted from the detailed TalkOrigins "Transitional
Vertebrate Fossils FAQ" (1994-1997) by Kathleen Hunt, and
updated from the book Evolution: What the Fossils Say and Why It
Matters by paleontologist / geologist Donald R. Prothero
(Columbia Univ Press, 2007).
 Cladoselache (late Devonian) --
Magnificent early shark fossils
Cladoselache (late Devonian) --
Magnificent early shark fossils Acanthodians (Silurian) -- A
puzzling group of spiny fish with similarities to early bony fish
Acanthodians (Silurian) -- A
puzzling group of spiny fish with similarities to early bony fish Osteolepis (mid-Devonian) -- One of the earliest crossopterygian
lobe-finned fishes, sharing characters with the lungfish, had paired
fins with a leg-like arrangement of major limb bones, capable of flexing
at the "elbow" and had an early-amphibian-like skull and teeth
Osteolepis (mid-Devonian) -- One of the earliest crossopterygian
lobe-finned fishes, sharing characters with the lungfish, had paired
fins with a leg-like arrangement of major limb bones, capable of flexing
at the "elbow" and had an early-amphibian-like skull and teeth
 Transitions among amphibians
Transitions among amphibians Picture
to the left is an early archosaur, Protosuchus, approx one meter
long, an early Triassic
Protosuchid crocodile. The Protosuchia retain long limbs and probably
were basically terrestrial in habit, but they resemble modern crocodiles
more closely than sphenosuchids in their general appearance and
configuration of the skull (from Carroll, page 280-281).
Picture
to the left is an early archosaur, Protosuchus, approx one meter
long, an early Triassic
Protosuchid crocodile. The Protosuchia retain long limbs and probably
were basically terrestrial in habit, but they resemble modern crocodiles
more closely than sphenosuchids in their general appearance and
configuration of the skull (from Carroll, page 280-281). All of these transitional animals lived
during the same brief 500,000 years. Before this site was studied, these
dinosaur groups were known from the much larger Judith River Formation,
where the fossils showed 5 million years of evolutionary stasis,
followed by the apparently abrupt appearance of the new forms. It turns
out that the sea level rose during that 500,000 years, temporarily
burying the Judith River Formation under water, and forcing the dinosaur
populations into smaller areas such as the site in Montana. While the
populations were isolated in this smaller area, they underwent rapid
evolution. When sea level fell again, the new forms spread out to the
re-exposed Judith River landscape, thus appearing "suddenly"
in the Judith River fossils, with the transitional fossils only existing
in the Montana site.
All of these transitional animals lived
during the same brief 500,000 years. Before this site was studied, these
dinosaur groups were known from the much larger Judith River Formation,
where the fossils showed 5 million years of evolutionary stasis,
followed by the apparently abrupt appearance of the new forms. It turns
out that the sea level rose during that 500,000 years, temporarily
burying the Judith River Formation under water, and forcing the dinosaur
populations into smaller areas such as the site in Montana. While the
populations were isolated in this smaller area, they underwent rapid
evolution. When sea level fell again, the new forms spread out to the
re-exposed Judith River landscape, thus appearing "suddenly"
in the Judith River fossils, with the transitional fossils only existing
in the Montana site.
 Probainognathus (mid-Triassic, 239-235 Ma, Argentina) -- Larger
brain with various skull changes: pineal foramen ("third eye")
closes, fusion of some skull plates
Probainognathus (mid-Triassic, 239-235 Ma, Argentina) -- Larger
brain with various skull changes: pineal foramen ("third eye")
closes, fusion of some skull plates
 Picture to the left is the famous "Archaeopteryx" --
a
transitional link between reptiles and birds -- there are 10 known specimens
(some are just feathers, some quite complete) -- see the detailed TalkOrigins
Picture to the left is the famous "Archaeopteryx" --
a
transitional link between reptiles and birds -- there are 10 known specimens
(some are just feathers, some quite complete) -- see the detailed TalkOrigins  Here we come to the most famous general
lineage of all, the horse sequence. It was the first such lineage to be
discovered, in the late 1800's, and thus became the most famous. It is a
very good sequence that has grown only more detailed and complete over
the years, changing mainly by the addition of large side-branches.
Here we come to the most famous general
lineage of all, the horse sequence. It was the first such lineage to be
discovered, in the late 1800's, and thus became the most famous. It is a
very good sequence that has grown only more detailed and complete over
the years, changing mainly by the addition of large side-branches. Samotherium (late Miocene) -- another
short-necked giraffe, and then split into Okapia (one species is
still alive, the okapi, essentially a living Miocene short-necked
giraffe)
Samotherium (late Miocene) -- another
short-necked giraffe, and then split into Okapia (one species is
still alive, the okapi, essentially a living Miocene short-necked
giraffe)


 Picture to the left is an intermediate Sirenian fossil: a seacow
with legs. Reconstructed skeleton of Pezosiren portelli. Length
is approximately 7 feet. Gray coloring represents extant fossils, white
elements are partly conjectural. [ Source: Domning, D. P. (2001) "The
earliest known fully quadrupedal sirenian" Nature 413:
625-627 ]
Picture to the left is an intermediate Sirenian fossil: a seacow
with legs. Reconstructed skeleton of Pezosiren portelli. Length
is approximately 7 feet. Gray coloring represents extant fossils, white
elements are partly conjectural. [ Source: Domning, D. P. (2001) "The
earliest known fully quadrupedal sirenian" Nature 413:
625-627 ]

 << If the First Adam is a myth, so is the
Second Adam. Think about that long and hard....If a scientist were to commit sacrilege and examine a
consecrated Host, what do you think he would tell you about it? Yet
you know he is absolutely, irrefutably wrong. Why, then, is he so
infallibly right when it comes to the creation of the Universe and of
man? Has not God told you just as plainly -- just as literally! -- of
how He miraculously created the Universe and man, as He has told you
of the miracle of Transubstantiation? Science refutes them both. Why,
then, do you believe Him on the one and doubt Him on the other?
Because of science? What kind of faith is that? And where is the
consistency? >>
<< If the First Adam is a myth, so is the
Second Adam. Think about that long and hard....If a scientist were to commit sacrilege and examine a
consecrated Host, what do you think he would tell you about it? Yet
you know he is absolutely, irrefutably wrong. Why, then, is he so
infallibly right when it comes to the creation of the Universe and of
man? Has not God told you just as plainly -- just as literally! -- of
how He miraculously created the Universe and man, as He has told you
of the miracle of Transubstantiation? Science refutes them both. Why,
then, do you believe Him on the one and doubt Him on the other?
Because of science? What kind of faith is that? And where is the
consistency? >>